| Identification | Back Directory | [Name]
Triphosphopyridine nucleotide | [CAS]
53-59-8 | [Synonyms]
TPN
TPNH
NADP
NADPH
Co II
NADP-ox
beta-tpn
BETA-NADP
cozymaseii
COENZYME II
nadphosphate
codehydraseii
NADP-oxidized
ATRAZINE NEAT
tpn(nucleotide)
nadide phosphate
CODEHYDROGENASE II
Triphosphopyridine n
Triphosphopyridinnucleotid
Triphosphopyridine nucleotid
TRIPHOSPHOPYRIDINE NUCLEOTIDE
CHLOROETHANE 200UG/ML IN METHANOL
BOTTLE 2,5L SMALL BROWN GLASS DIN45
nicotinamide adenine dinucleotide-P
NICOTINAMIDE ADENINE DINUCLEOTIDE PHOSPHATE
BETA-NICOTINAMIDE ADENINE DINUCLEOTIDE PHOSPHATE
B-NICOTINAMIDEADENINEDINUCLEOTIDEPHOSPHATE,HYDRATE
B-NICOTINAMIDE ADENINE DINUCLEOTIDE*PHOS PHATE FREE
β-Nicotinamide adenine dinucleotide phosphate (NADP)
β-nicotinamide adenine dinucleotide phosphate hydrate
BETA-NICOTINAMIDE ADENINE DINUCLEOTIDE PHOSPHORIC ACID
BETA-NICOTINAMIDE ADENINE DINUCLEOTIDE PHOSPHATE REDUCED
BETA-NICOTINAMIDE ADENINE DINUCLEOTIDE PHOSPHATE
(NADP+)
NADP(β-Nicotinamide adenine dinucleotide phosphate hydrate)
β-nicotinamide adenine dinucleotide phosphate(NADP+) free base
NADP (b-NicotinaMide adenine dinucleotide phosphate)trihydrate
Beta-nicotinamide adenine dinucleotide phosphate(NADP+) free base
β-NADP, Coenzyme II, NADP, TPN, Triphosphopyridine nucleotide
enosine2’-(dihydrogenphosphate)5’-(trihydrogenpyrophosphate),innersalt
2’-(dihydrogenphosphate),5’.fwdarw.’-adenosine5’-(trihydrogendiphosphate
5'-ester with3-(aminocarbonyl)-1-b-D-ribofuranosylpyridinium, inner salt
Adenosine5'-(trihydrogen diphosphate), 2'-(dihydrogen phosphate), P'®
β-Nicotinamide Adenine Dinucleotide Phosphate [for Biochemical Research]
pyridinium,3-carbamoyl-1-beta-d-ribofuranosyl-,hydroxide,5’,5’-esterwithad
esterwith3-(aminocarbonyl)-1-beta-d-ribofuranosylpyridiniumhydroxide,inner
beta-NicotinaMide Adenine Dinucleotide Phosphate [for BiocheMical Research]
β-Nicotinamide adenine dinucleotide phosphate hydrate,β-NADP, CoenzymeII, NADP, TPN, Triphosphopyridine nucleotide
[(2R,3R,4R,5R)-2-(6-aminopurin-9-yl)-5-[[[[(2R,3S,4R,5R)-5-(5-carbamoylpyridin-1-yl)-3,4-dihydroxy-oxolan-2-yl]methoxy-hydroxy-phosphoryl]oxy-hydroxy-phosphoryl]oxymethyl]-4-hydroxy-oxolan-3-yl]oxyphosphonic acid | [EINECS(EC#)]
200-178-1 | [Molecular Formula]
C21H28N7O17P3 | [MDL Number]
MFCD00067537 | [MOL File]
53-59-8.mol | [Molecular Weight]
743.41 |
| Hazard Information | Back Directory | [Description]
Triphosphopyridine nucleotide is a coenzyme composed of ribosylnicotinamide 5'-phosphate (NMN) coupled by pyrophosphate linkage to the 5'-phosphate adenosine 2',5'-bisphosphate. Triphosphopyridine nucleotide serves as an electron carrier in a number of reactions, being alternately oxidized (NADP+) and reduced (NADPH). | [Chemical Properties]
Triphosphopyridine nucleotide is white or off-white powder, it is easy to absorb moisture and deliquescence. pKa{1}=3.9; pKa{2}=6.1. It is soluble in water, methanol, insoluble in ethanol, insoluble in ether and ethyl acetate. | [Uses]
β-Nicotinamide adenine dinucleotide phosphate hydrate is suitable for use in:
the measurement of Glucose-6-phosphate dehydrogenase activity
the Cytochrome P450 3A4 assay as a part of NADPH-regenerating system
the Cytochrome P450 2D6 assay as a part of NADPH-regenerating system
the determination of Glucose-6-phosphate content | [Biological Functions]
Triphosphopyridine nucleotide (NADP) serves as an electron carrier in a number of reactions, being alternately oxidized (NADP+) and reduced (NADPH). | [Biochem/physiol Actions]
β-Nicotinamide adenine dinucleotide 2′-phosphate (NADP+) and β-Nicotinamide adenine dinucleotide 2′-phosphate, reduced (NADPH) comprise a coenzyme redox pair (NADP+:NADPH) involved in a wide range of enzyme catalyzed oxidation reduction reactions. The NADP+/NADPH redox pair facilitates electron transfer in anabolic reactions such as lipid and cholesterol biosynthesis and fatty acyl chain elongation. The NADP+/NADPH redox pair is used in a variety of antioxidation mechanism where it protects agains reactive oxidation species accumulation. NADPH is generated in vivio by the pentose phosphate pathway (PPP). | [Synthesis]
Triphosphopyridine nucleotide is prepared by the reaction of NADPH. It is synthesised mainly by the interaction of both NfrA1 enzyme and a Bacillus subtilis under the conditions of bacterial luciferase. Reaction conditions were as follows: with hydrogenchloride; NfrA1 enzyme; nitrofurazone; 2-amino-2-hydroxymethyl-1,3-propanediol In water at 23℃; pH=7.0; Enzyme kinetics; Further Variations; Reagents; Oxidation.
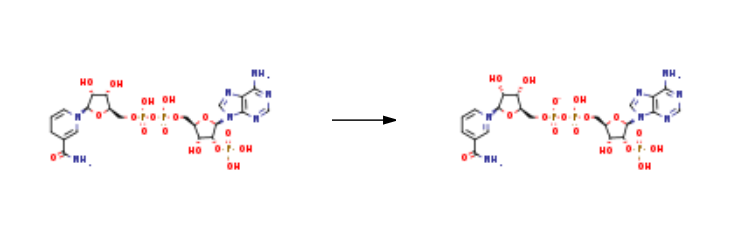 | [Purification Methods]
Purify NMN by passage through a column of Dowex-1 (Clform) and washing with H2O until no absorbance is observed at 260 nm. The tubes containing NMN are pooled, adjusted to pH 5.5-6 and evaporated in vacuo to a small volume. This is adjusted to pH 3 with dilute HNO3 in an ice-bath and treated with 20volumes of Me2CO at 0-5o. The heavy white precipitate is collected by centrifugation at 0o. It is best stored wet and frozen or it can be dried to give a gummy residue. It has max 266nm ( 4,600) and min 249nm ( 3600) at pH 7.0 (i.e. no absorption at 340nm). It can be estimated by reaction with CNor hydrosulfite which form the 4-adducts (equivalent to NADH) which have UV max 340nm ( 6,200). Thus after reaction, an OD340 of one is obtained from a 0.1612mM solution in a 1cm path cuvette. [Plaut & Plaut Biochemical Preparations 5 56 1957, Maplan & Stolzenbach Methods Enzymol 3 899 1957, Kaplan et al. J Am Chem Soc 77 815 1955, Beilstein 22/2 V 168.] |
|
|