| Identification | Back Directory | [Name]
MOTILIN (HUMAN, PORCINE) | [CAS]
52906-92-0 | [Synonyms]
motilin
MOTILIN, PORCINE
MOTILIN PORCINE ≥97%
FVPIFTYGELQRMQEKERNKGQ
Motilin, porcine, ≥97% (HPLC)
Gastricmotorstimulatorypeptide
Motilin (26-47), human, porcine
GASTRIC MOTOR STIMULATORY PEPTIDE, PORCINE
PHE-VAL-PRO-ILE-PHE-THR-TYR-GLY-GLU-LEU-GLN-ARG-MET-GLN-GLU-LYS-GLU-ARG-ASN-LYS-GLY-GLN
H-PHE-VAL-PRO-ILE-PHE-THR-TYR-GLY-GLU-LEU-GLN-ARG-MET-GLN-GLU-LYS-GLU-ARG-ASN-LYS-GLY-GLN-OH | [Molecular Formula]
C120H188N34O35S | [MDL Number]
MFCD00076521 | [MOL File]
52906-92-0.mol | [Molecular Weight]
2699.05 |
| Questions And Answer | Back Directory | [Gene, mRNA, and precursor]
The human MLN gene is mapped to 6p21.31. It is a single copy gene composed of five exons, spanning approximately 9 kb. The 22-aa MLN peptide gene has a
structure similar to that of ghrelin, and is encoded by
exon 3 and part of exon 2 . The aa sequences
of preproMLN from the pig, rabbit, human, sheep, and
monkey have been deduced from the cDNA sequence.
The C-terminal MAP is largely encoded by parts of exons
3 and 4, with the last two aa of the MLN precursor and the
30 untranslated region encoded by exon 5. MLN is identified throughout the gastrointestinal (GI)
tract of numerous species, and is found predominantly
not only in the endocrine M cells of the duodenal mucosa
but also in the myenteric plexus, where it is colocalized
with neurons immunoreactive for neuronal nitric oxide
synthase. MLN-producing cells decrease distally in the
small intestine. MLN is also present in the thyroid and
the brain, where the highest concentration is found in
the hypothalamus. | [Synthesis and release]
MLN is released in circulation at approximately 100-
min intervals in the interdigestive state, and the ingestion
of food during this period prevents the secretion of
MLN. The cholinergic pathway is an important regulator of the release of MLN. Muscarinic3 (M3) receptors
have been found responsible for MLN release from
canine MLN cells in the perifusion system. Exogenous
MLN treatment stimulates endogenous MLN release
through the muscarinic receptors on MLN-producing
cells via preganglionic pathways involving
5-hydroxytryptamine 3 receptors. | [Receptors]
Feighner and colleagues first identified the orphan
GPCR, GPR38, as the human MLN receptor (MTLR,
MLNR), for which two alternatively spliced forms exist. An mRNA, GPR38-A (splice variant 1a),
encodes a 412-aa protein with seven predicted α-helical
transmembrane domains, the hallmark feature of GPCRs,
and is an active form of the receptor, whereas GPR38-B
(variant 1b) mRNA encodes a 386-aa protein with five
predicted transmembrane domains. The signal transduction pathway of MLNR is
unknown. However, the molecular and cellular mechanisms involved in MLN-induced MLNR desensitization
have been observed. After MLN stimulation, the MLNR
becomes phosphorylated, probably via GPCR kinases.
This leads to the recruitment of β-arrestin-2, which targets
the receptor to clathrin-coated pits. Upon internalization,
the β-arrestin dissociates from the receptor, and the MLN receptor complex is subsequently sorted to the recycling
endosomes that transport the MLNR back to the plasma
membrane. | [Agonists]
Erythromycin is extensively used as an effective agent
to accelerate the gastric emptying of food in patients with
diabetic gastroparesis through MLNR. Several pharmaceutical companies have generated MLN-like macrolides
(motilides) that are erythromycin derivatives devoid of
antibiotic activity but with strong affinity to MLNR. However, the first drugs, EM-523 and its successor EM-574,
failed because of their chemical instability and low bioavailability. Another compound, ABT-229, was also
unsuccessful for treating functional dyspepsia and diabetic gastroparesis, possibly because of its strong desensitizing properties. To overcome these limitations, the
second-generation compounds were developed. Based
on the N-terminal region of MLN and its biological activity, a novel synthetic human MLN analog, atilmotin,
which lacks the C-terminal end, was developed to accelerate gastric emptying in healthy subjects. However, its
effect was poor and only detected during the first
30min. An acid-resistant nonpeptidyl MLN agonist,
mitemcinal, was developed that is orally active and could
be beneficial for the treatment of delayed gastric emptying
and transit. However, symptom relief occurred only in a
subset of patients. Two MLNR agonists, RQ-00201894 and
camicinal (GSK962040), have been developed.
RQ-00201894, a novel nonmacrolide MLN agonist, demonstrates agonistic activity similar to that of MLN in
human MLNR expressed in CHO cells, and facilitates cholinergically mediated human antral muscle contractions
evoked by electrical field stimulation. However, its structure remains unknown. Camicinal, in contrast, is
derived from a benzylpiperazine molecular structure that
selectively activates the recombinant human MLNR,
induces contractions in human and rabbit isolated stomach preparations, and increases the fecal output in conscious rabbits. | [Antagonists]
The macrocyclic peptidomimetic TZP-201 is a potent
semisynthetic nonpeptidyl MLN antagonist that has been
326 30B. Motilin
I-3. Gastrointestinal hormones
used for the treatment of various forms of moderate to
severe diarrhea associated with irritable bowel syndrome, cancer, and infectious diseases. Preclinical data
show that TZP-201 is efficacious in a dog model of
chemotherapy-induced diarrhea. Similarly, an orally
active MLNR antagonist, MA-2029, inhibits MLN induced GI motility without affecting the basal GI tone
or gastric emptying rate. | [Biological functions]
MLNR is mainly found in the GI tract, but the exact
localization is species-dependent. In humans, through
binding experiments with iodinated porcine [Leu13]
MLN, the MTLR density was found to be the highest in
the gastroduodenal region, and decreased distally in
the small intestine toward the colon. MLNR immunoreactivity is present in muscle cells and the myenteric
plexus, but not in mucosal or submucosal cells, in
humans. In rabbits, the highest MLNR density is found
in the colon. MLNR has been found outside the GI tract
in the hypothalamus, nodose ganglion, thyroid, and bone
marrow. Mlnr gene expression has been found in the
lung and heart in Suncus murinus, suggesting that MLN
could have an unknown function in the respiratory and
cardiovascular systems.The main biological functions are to increase lower esophageal sphincter (LES) pressure and to induce the interdigestive motor complex (IMC) for removing debris and
cleaning the GI tract. Recent studies in humans show that
MLN-induced gastric contractions stimulate hunger. | [Clinical implications]
Plasma MLN concentrations increase significantly in
patients with diabetic gastroparesis who maintain a normal migrating motor complex (MMC), even without
antral phase-III activity. Similarly, a higher plasma
MLN concentration is reported in hypergastrinemic
chronic atrophic gastritis and chronic renal failure,
whereas decreased MLN release has been observed in
patients with functional bowel disorders such as chronic
idiopathic constipation or idiopathic megacolon. Abnormal fluctuations of MLN also occur with severe pancreatic
insufficiency. Fasting and postprandial levels of MLN are
significantly raised in patients with infectious diarrhea.
Hypermotilinemia is often associated with Crohn’s disease, ulcerative colitis, and tropical malabsorption. |
| Chemical Properties | Back Directory | [storage temp. ]
−20°C
| [solubility ]
25mg/mL in DMSO & DMF, slightly soluble in ethanol | [form ]
Solid | [color ]
White to off-white | [Water Solubility ]
Soluble to 1 mg/ml in water |
| Hazard Information | Back Directory | [Description]
Motilin was isolated from the duodenojejunal mucosa and
found to control the motor activity of the digestive tract. It is
a potential therapeutic drug target for improving digestive
dysmotility. Motilin was isolated from a side fraction produced
during the purification of secretin in 1971 and was
found to stimulate contractility in the fundus of the stomach. Complete porcine and human motilin were purified
and sequenced in 1973 and 1983 respectively. | [General Description]
Motilin is a 22-residue polypeptide isolated from the duodenum.Its secretion is stimulated by the presence of acid inthe duodenum. Motilin inhibits gastric motor activity anddelays gastric emptying. | [Clinical Use]
To date, almost all macrolides have lacked effectiveness as MLN agonists. However, pharmacies have been
trying to develop new types of macrolides, such as
PF-04548043 (formerly known as KOS-2187), for the treatment of GI motility disorders such as gastroparesis and
gastroesophageal reflux disease. Moreover, the new
MLNR agonist RQ-00201894 is a promising drug for
the treatment of gastroparesis, postoperative ileus, and
functional dyspepsia. | [storage]
Store at -20°C | [Structure and conformation]
Motilin is highly conserved across species, and is synthesized as a part of a larger inactive prohormone. Structure-activity studies with analogs and fragments of porcine motilin have shown an N-terminal region, which is considered a
physiological and biological active site, and a C-terminal
α-helical domain. Human motilin is synthesized as a preprohormone composed of 133 aa residues, each consisting
of a 25-aa signal peptide followed by a 22-aa (mature motilin) and a C-terminal motilin-associated peptide (MAP). In the N-terminal region, identical aa sequences exist
in human and porcine motilin, but differ from canine
motilin at positions 7, 8, 12, 13, and 14 . Chicken MLN also differs from human and porcine
sequences by six residues at positions 4, 7–10, and
12, and the binding affinity and pharmacological
potency against the chicken MLNR differ from those
for mammalian MLN.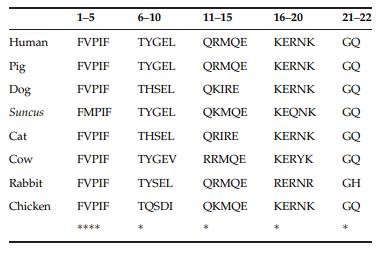 |
|
|