| Identification | Back Directory | [Name]
N-2-AMINOETHYL HOMOPIPERIDINE | [CAS]
51388-00-2 | [Synonyms]
BUTTPARK 154\16-20
RARECHEM AL BW 1126
2-AZEPAN-1-YLETHANAMINE
2-AZEPAN-1-YL-ETHYLAMINE
2-(1-azepanyl)ethanamine
2-(AZEPAN-1-YL)ETHAN-1-AMINE
2-(1-azepanyl)ethanamine 1HCl
N-2-AMINOETHYL HOMOPIPERIDINE
hexahydro-1H-Azepine-1-ethanamine
1H-Azepine-1-ethanamine, hexahydro-
2-(1-azepanyl)ethanamine(SALTDATA: HCl) | [Molecular Formula]
C8H18N2 | [MDL Number]
MFCD00046120 | [MOL File]
51388-00-2.mol | [Molecular Weight]
142.24 |
| Chemical Properties | Back Directory | [Melting point ]
200 °C | [Boiling point ]
46-52°C/1mm | [density ]
0.908±0.06 g/cm3(Predicted) | [pka]
10.21±0.10(Predicted) | [InChI]
InChI=1S/C8H18N2/c9-5-8-10-6-3-1-2-4-7-10/h1-9H2 | [InChIKey]
QHRBDFUMZORTQD-UHFFFAOYSA-N | [SMILES]
N1(CCN)CCCCCC1 |
| Hazard Information | Back Directory | [Uses]
2-Azepan-1-yl-ethylamine is an important organic reagent that can be used as a building block to synthesize mang organic compounds,such as 1-[2-(azepan-1-yl)ethyl]-3-phenylurea. | [Synthesis]
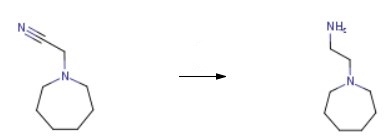
A mixture of 2-(azepan-1-yl)acetonitrile (5.58 g, 40.4 mmol) and Raney Nickel (50% w/w in water, ca. 5 mL) in EtOH (50 mL) and cNH3 (4 mL) was stirred under H2 (60 psi) for 16 h. The mixture was filtered through Celite, washed with EtOH (3 x 10 mL) and the solvent evaporated to give crude 2-Azepan-1-yl-ethylamine as an oil.
 |
|
|