| Identification | Back Directory | [Name]
Dimethyl Furan-2,5-dicarboxylate | [CAS]
4282-32-0 | [Synonyms]
Dimethyl 2,5-furandic
2,5-Bis(methoxycarbonyl)furan
Methyl Furan-2,5-dicarboxylate
Dimethy Furan-2,5-dicarboxylate
Dimethyl 2,5-furandicarboxylate
Dimethyl furan dicarboxylic acid
dimethyl furan-2,5-dicarboxylate
2,5-Dimethyl 2,5-furandicarboxylate
Dimethyl furan-2,5-dicarboxylate 99%
DimethylFuran2,5-Dicarboxylate (FDCAE)
2,5-Furandicarboxylic acid dimethyl ester
Furan-2,5-dicarboxylic acid dimethyl ester
2,5-Furandicarboxylic acid, 2,5-dimethyl ester | [EINECS(EC#)]
248-451-4 | [Molecular Formula]
C8H8O5 | [MDL Number]
MFCD00092317 | [MOL File]
4282-32-0.mol | [Molecular Weight]
184.15 |
| Chemical Properties | Back Directory | [Melting point ]
112°C | [Boiling point ]
278.08°C (rough estimate) | [density ]
1.3840 (rough estimate) | [refractive index ]
1.5690 (estimate) | [storage temp. ]
Sealed in dry,2-8°C | [form ]
crystals | [color ]
White |
| Hazard Information | Back Directory | [Description]
Dimethyl Furan-2,5-dicarboxylate is a structural analog of diacids. It has been synthesized by the reaction of 5-hydroxymethylfurfural with ethyl esters and phosphotungstic acid. | [Uses]
2,5-Furandicarboxylic acid dimethyl ester can be used as organic synthesis intermediates and pharmaceutical intermediates, mainly used in laboratory research and development processes and chemical production processes. | [Synthesis]
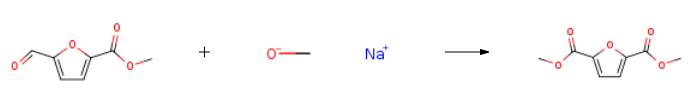 Preparation of dimethyl FDCA from methyl 5-formyl-2- furoate: A 15 niL glass liner was charged with a magnetic stirring bar, methyl 5- formyl-2-furoate (117 mg, 0.76 mmol), methanol (10 mL) and sodium methoxide (4 mg, 0.076 mmol) to give a clear solution. A 1.2 wt% Au/Ti02 (41.5 mg, 2.53 |ì|é|?|? Au) catalyst was added to give a purple suspension and the vial was placed in a 75 mL Parr Hastelloy C-276 reactor. The reactor was closed and flushed 3x with compressed air and then pressurized at 4.6 bar. Stirring was started (600 rpm) and the reaction was allowed to proceed at room temperature. After 19 h, the reaction had consumed 0.25 bar of air and the reactor was opened. The reaction mixture was filtered over Celite to remove the catalyst, which was washed with a httle methanol and dichlorom ethane. The combined organic layers were washed with water, dried over MgS04, filtered, and the solvent was removed under reduced pressure. 2,5-FDCA dimethyl ester was obtained as light yellow crystals (106 mg, yield 76%). Preparation of dimethyl FDCA from methyl 5-formyl-2- furoate: A 15 niL glass liner was charged with a magnetic stirring bar, methyl 5- formyl-2-furoate (117 mg, 0.76 mmol), methanol (10 mL) and sodium methoxide (4 mg, 0.076 mmol) to give a clear solution. A 1.2 wt% Au/Ti02 (41.5 mg, 2.53 |ì|é|?|? Au) catalyst was added to give a purple suspension and the vial was placed in a 75 mL Parr Hastelloy C-276 reactor. The reactor was closed and flushed 3x with compressed air and then pressurized at 4.6 bar. Stirring was started (600 rpm) and the reaction was allowed to proceed at room temperature. After 19 h, the reaction had consumed 0.25 bar of air and the reactor was opened. The reaction mixture was filtered over Celite to remove the catalyst, which was washed with a httle methanol and dichlorom ethane. The combined organic layers were washed with water, dried over MgS04, filtered, and the solvent was removed under reduced pressure. 2,5-FDCA dimethyl ester was obtained as light yellow crystals (106 mg, yield 76%). |
|
|