| Identification | Back Directory | [Name]
1-Benzyl-3-ethoxycarbonyl-4-piperidone | [CAS]
41276-30-6 | [Synonyms]
-3-ethoxycarbonyL
LABOTEST-BB LT00440756
1-Benzyl-3-carbethoxy-4-piperidone
1-BENZYL-3-CARBOETHOXY-4-PIPERIDONE
N-BENZYL-3-CARBOETHOXY-4-PIPERIDONE
-Benzyl-3-ethoxycarbonyl-4-piperidone
1-BENZYL-3-ETHOXYCARBONYL-4-PIPERIDONE
ethyl 1-benzyl-4-oxopiperidine-3-carboxylate
1-(benzyl)-4-keto-nipecotic acid ethyl ester
ethyl 4-oxo-1-(phenylmethyl)piperidine-3-carboxylate
Ethyl 1-benzyl-4-oxopiperidine-3-carboxylate(HCl ForM)
1-BENZYL-4-OXO-PIPERIDINE-3-CARBOXYLIC ACID ETHYL ESTER
4-oxo-1-(phenylmethyl)-3-piperidinecarboxylic acid ethyl ester
3-Piperidinecarboxylic acid, 4-oxo-1-(phenylmethyl)-, ethyl ester | [Molecular Formula]
C15H19NO3 | [MDL Number]
MFCD00792540 | [MOL File]
41276-30-6.mol | [Molecular Weight]
261.32 |
| Chemical Properties | Back Directory | [Boiling point ]
379.7±42.0 °C(Predicted) | [density ]
1.154±0.06 g/cm3(Predicted) | [storage temp. ]
under inert gas (nitrogen or Argon) at 2-8°C | [pka]
10.77±0.20(Predicted) |
| Questions And Answer | Back Directory | [Synthesis]
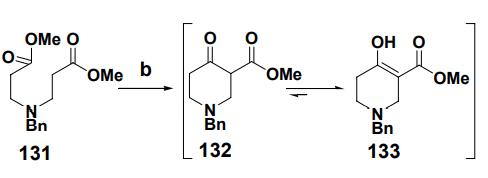
To a suspension of NaH (5.38 g, 0.13 mol) in
benzene, a solution of Michael adduct 131 (25 g,
0.09 mol) in PhH was added drop-wise and
refluxed for 3 hours. The reaction mixture was
allowed to cool and quenched with saturated
NH4Cl solution, extracted with ethyl acetate, washed with water, brine, dried over
anhydrous Na2SO4, filtered and concentrated in vacuo. Column chromatographic
separation over silica gel (EtOAc- pet ether) afforded ketoester, as a pale yellow oil (18.15
g).
|
|
|