| Identification | Back Directory | [Name]
(+)-N,N-BIS[(1S)-1-PHENYLETHYL]-DINAPHTHO[2,1-D:1',2'-F][1,3,2]DIOXAPHOSPHEPIN-4-AMINE, (11BR) | [CAS]
380230-02-4 | [Synonyms]
3,4-a'
(S,S,S)-(+)-(3,5-DIOXA-4-PHOSPHA-CYCLOHE
]dinaphthalen-4-yl)bis(1-phenylethyl)amine
]dinaphthalen-4-yl)bis[(1S)-1-phenylethyl]amine.
3,4-a']dinaphthalen-4-yl)bis[(1S)-1-phenylethyl]amine
3,4-a']dinaphthalen-4-yl)bis[(1S)-1-phenylethyl]amine,95%
(S,S,S)-(+)-(3,5-Dioxa-4-phosphacyclohepta[2,1-a:3,4-a’
3,4-a']dinaphthalen-4-yl)bis[(1S)-1-phenylethyl]amine,min.95%
3,4-a']dinaphthalen-4-yl)bis[(1S)-1-phenylethyl]amine, min. 95%
N,N-Bis((S)-1-phenylethyl)dinaphtho-[2,1-d:1',2'-f][1,3,2]dioxaphosphepin-4-amine
N,N-Bis[(1S)-1-phenylethyl]-dinaphtho[2,1-d:1',2'-f][1,3,2]dioxaphosphepin-4-amine
(+)-N,N-BIS[(1S)-1-PHENYLETHYL]-DINAPHTHO[2,1-D:1',2'-F][1,3,2]DIOXAPHOSPHEPIN-4-AMINE, (11BR)
(S,S,S)-(+)-(3,5-DIOXA-4-PHOSPHA-CYCLOHEPTA[2,1-A:3,4-A']DINAPHTHALEN-4-YL)BIS(1-PHENYLETHYL)AMINE
(S)-(+)-(3,5-DIOXA-4-PHOSPHA-CYCLOHEPTA[2,1-A:3,4-A']DINAPHTHALEN-4-YL)BIS[(1S)-1-PHENYLETHYL]AMINE
(S,S,S)-(+)-(3,5-Dioxa-4-phosphacyclohepta[2,1-a:3,4-a']dinaphthalen-4-yl)bis(1-phenylethyl)aMine 97%
S)-(+)-(3,5-Dioxa-4-phospha-cyclohepta[2,1-a:3,4-a']dinaphthalen-4-yl)bis[(1S)-1-phenylethyl]amine,min.95%
(S)-(+)-(3,5-Dioxa-4-phospha-cyclohepta[2,1-a:3,4-a']dinaphthalen-4-yl)bis[(1S)-1-phenylethyl]amine, min. 95% | [Molecular Formula]
C36H30NO2P | [MDL Number]
MFCD04117688 | [MOL File]
380230-02-4.mol | [Molecular Weight]
539.6 |
| Chemical Properties | Back Directory | [Melting point ]
88-89 °C
| [alpha ]
+13.1° (c 1.01, CHCl3) | [Boiling point ]
710.7±63.0 °C(Predicted) | [storage temp. ]
under inert gas (nitrogen or Argon) at 2-8°C | [form ]
Powder | [pka]
-0.57±0.20(Predicted) | [color ]
off-white | [Sensitive ]
moisture sensitive | [InChIKey]
LKZPDRCMCSBQFN-UIOOFZCWSA-N |
| Questions And Answer | Back Directory | [Reaction]
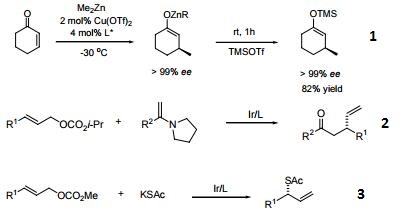
- A ligand for asymmetric conjugate addition of dialkyl zinc reagents to activated olefins.
- Iridium-catalyzed regioselective and enantioselective allylation of enamines.
- Iridium-catalyzed asymmetric allylation of KSAc.
|
| Hazard Information | Back Directory | [Uses]
The product may be used as a ligand in:
- Iridium-catalyzed allylic etherification of acyclic, achiral allylic carbonates with potassium silanolates to form chiral allylic alcohols.
- Palladium-catalyzed asymmetric allylic cyclisation of N-tosyl and N-benzyl carbonates to form the corresponding pyrrolidine and piperidine derivatives, respectively.
- Intramolecular iridium-catalyzed allylic cyclizationof (E)-allylic methyl carbonates to form 2,5-trans/cis pyrrolidine derivatives.
|
|
| Company Name: |
LaaJoo Gold
|
| Tel: |
021-60702684 18516024827 |
| Website: |
http://www.approvedhomemanagement.com/ShowSupplierProductsList20079/0.htm |
| Company Name: |
Energy Chemical
|
| Tel: |
021-021-58432009 400-005-6266 |
| Website: |
http://www.energy-chemical.com |
|