| Identification | Back Directory | [Name]
5-(4-phenoxyphenyl)-7H-pyrrolo[2,3-d]pyriMidin-4-ylaMine | [CAS]
330786-24-8 | [Synonyms]
ibrutinib N-2
Ibrutinib INT1
Ilutinib impurity 8
Ibrutinib Impurity 8
Ibrutinib Impurity 46
3-d]pyriMidin-4-ylaMine
Ibrutinib intermediate 2
Ibrutinib intermeidate N-2
Ibrutinib intermediates N-2
Ibrutinib deacryloylpiperidine
5-(4-phenoxyphenyl)-7H-pyrrolo[2
Ibrutinib N-Despiperidinyl Impurity
IbrutinibintermeidChemicalbookateN-2
PCI-32765 (IBRUTINIB) INTERMEDIATE(N-2)
3-(4-phenoxyphenyl)-1H-pyrazolo[3,4-d]pyriMidine
5-(4-phenoxyphenyl)-7H-pyrrolo[2,3-d]pyriMidin-4-y
Ibrutinib deacryloylpiperidine (Ibrutinib Impurity)
3-(4-Phenoxyphenyl)pyrazolo[3,4-D]pyrimidin-4-amine
10-Morpholineethanamine,N-(cyclohexylcarbonimidoyl)-
5-(4-Phenoxyphenyl)-7H-pyrrolo[2,3-d]pyriMidin-4-aMine
5-(3-Phenoxyphenyl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine
3-(4-Phenoxypheny)- 1Hpyrazolo[3.4-dlpyrimidin-4-amine
3-(4-phenoxyphenyl)-1H-pyrazolo[3,4-d]pyriMidin-4-aMine
3-(4-phenoxyphenyl)-2H-pyrazolo[3,4-d]pyrimidin-4-amine
-(4-phenoxyphenyl)-7H-pyrrolo[2,3-d]pyriMidin-4-ylaMine
4-aMino-3-(4-phenoxyphenyl)-1H-pyrazolo[3,4-d]pyriMidine
5-(4-phenoxyphenyl)-7H-pyrrolo[2,3-d]pyriMidin-4-ylaMine
1H-Pyrazolo[3,4-d]pyriMidin-4-aMine, 3-(4-phenoxyphenyl)-
3-(4-Phenoxy Phenyl)-1H-pyrazole [3,4-d]pyrimidin-4-Amine
2) 3-(4-PHENOXYPHENYL)-1H-PYRAZOLO[3,4-D]PYRIMIDIN-4-AMINE
3-(4-Phenoxyphenyl)-1H-pyrazoleand[3,4-d]pyrimidine-4-amine
5- (4-phenoxyphenyl) -7H-pyrrolio [2,3-d] pyrimidin-4-ylamine
3-(4-phenoxyphenyl)-1 H- pyrazolium [3,4- D] pyrimidine-4-amine
3-(4- phenoxyphenyl) -1H- pyrazolo [3,4-d] pyrimidine -4- amine
5-(4-phenoxyphenyl)-7H-pyrrolo[2,3-d]pyriMidin-4-ylamine (N-2)
3-(4-Phenoxyphenyl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine/Ibrutinib impurity
5-(4-phenoxyphenyl)-7H-pyrrolo[2,3-d]pyriMidin-4-ylaMine ISO 9001:2015 REACH
Ibrutinib impurity 3/
3-(4-Phenoxyphenyl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine
5-(4-PHENOXYPHENYL)-7H-PYRROLO[2,3-D]PYRIMIDIN-4-YLAMINE/3-(4-PHENOXYPHENYL)-1H-PYRAZOLO[3,4-D]PYRIMIDIN-4-AMINE | [EINECS(EC#)]
810-090-0 | [Molecular Formula]
C17H13N5O | [MDL Number]
MFCD28334242 | [MOL File]
330786-24-8.mol | [Molecular Weight]
303.318 |
| Chemical Properties | Back Directory | [Melting point ]
>262oC (dec.) | [Boiling point ]
577.4±50.0 °C(Predicted) | [density ]
1.380±0.06 g/cm3(Predicted) | [storage temp. ]
Keep in dark place,Inert atmosphere,Room temperature | [solubility ]
DMSO (Slightly), Ethanol (Slightly) | [form ]
Solid | [pka]
10.40±0.30(Predicted) | [color ]
Pale Brown to Brown | [InChI]
InChI=1S/C17H13N5O/c18-16-14-15(21-22-17(14)20-10-19-16)11-6-8-13(9-7-11)23-12-4-2-1-3-5-12/h1-10H,(H3,18,19,20,21,22) | [InChIKey]
YYVUOZULIDAKRN-UHFFFAOYSA-N | [SMILES]
C1=NC(N)=C2C(C3=CC=C(OC4=CC=CC=C4)C=C3)=NNC2=N1 |
| Hazard Information | Back Directory | [Description]
5-(4-phenoxyphenyl)-7H-pyrrolo[2,3-d]pyriMidin-4-ylaMine, also known as ibrutinib N-2, is a light brown solid. It can be prepared from 3-Iodo-1H-pyrazolo[3,4-d]pyrimidin-4-amine and 4-phenoxybenzeneboronicacid by Suzuki coupling reaction[1]. Ibrutinib N-2 is often used in the preparation of the marketed drug PCI-32765 (IMBRUVICAVR)[2]. PCI-32765 is aIbrutinib -BTK inhibitor and can be used as an anticancer drug to treat B cell cancers like mantle cell lymphoma and chronic lymphocytic leukemia. | [Uses]
As Ibrutinib intermediates, 5-(4-phenoxyphenyl)-7H-pyrrolo[2,3-d]pyriMidin-4-ylaMine can be used as organic synthesis intermediates and pharmaceutical intermediates, mainly used in laboratory research and development and chemical production processes. | [Application]
3-(4-Phenoxyphenyl)-1h-pyrazolo[3,4-d]pyrimidin-4-amine is an intermediate for the synthesis of ibrutinib, which is mainly used in drug synthesis and related experimental studies. | [Synthesis]
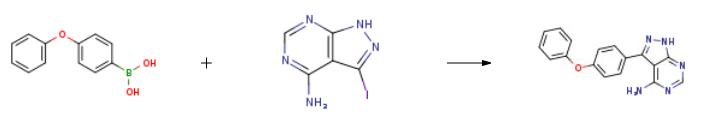
2 g of 3-iodo-4-aminopyrazolo[3,4-d]pyrimidine (7.7 mmol),3.28 g p-phenoxybenzeneboronic acid (15.4 mmol)And 5.28 g of K3PO4 (23.0 mmol) were dissolved in 25 mL of dioxane and 10 mL of water.After stirring for 5-8 minutes, argon gas was passed for 20 minutes.An additional 1.4 g of tetrakis(triphenylphosphine)palladium (1.2 mmol) was added.After heating again for 10 minutes, the heating was started, and the reaction was carried out at 120 ?? C for 24 hours.After the reaction was completed, it was cooled to room temperature, stirred for 24 hours to wait for product to precipitate, and the reaction mixture was washed with 25 mL of water and filtered.The filtered solid was again washed with 75 ml of methanol, washed with 50 mL of ethanol, and dried in a dry box.There was obtained 1.75 g of 3-(4-phenoxyphenyl)-1H-pyrazolo[3,4-d]pyrimidine-4-amine in a yield of 75%. | [References]
[1] Rampalli, S, et al. Process for the Preparation of Ibrutinib. PCT Int. Appl. 2017; 134588.
[2] Dasar G, et al. One-step synthesis of magnetically recyclable
palladium loaded magnesium ferrite
nanoparticles: application in synthesis of
anticancer drug PCI-32765. Inorganic and Nano-Metal Chemistry, 2020; 50. |
|
|