| Identification | Back Directory | [Name]
DICHLORO[(R)-(-)-4,12-BIS(DI(3,5-XYLYL)PHOSPHINO)-[2,2]-PARACYCLOPHANE][(1S,2S)-(-)-1,2-DIPHENYLETHYLENEDIAMINE]RUTHENIUM | [CAS]
325150-57-0 | [Synonyms]
DICHLORO[(R)-(-)-4,12-BIS(DI(3,5-XYLYL)PHOSPHINO)-[2,2]-PARACYCLOPHANE][(1S,2S)-(-)-1,2-DIPHENYLETHYLENEDIAMINE]RUTHENIUM
Dichloro[(R)-(-)-4,12-bis(di(3,5-xylyl)phosphino)-[2.2]-paracyclophane][(1S,2S)-(-)-1,2-diphenylethylenediamine]ruthenium(II)
DICHLORO[(R)-(-)-4,12-BIS(DI(3,5-XYLYL)PHOSPHINO)-[2.2]-PARACYCLOPHANE][(1S,2S)-(-)-1,2-DIPHENYLETHYLENEDIAMINE]RUTHENIUM (II)
Dichloro[(R)-(-)-4,12-bis(di(3,5-xylyl)phosphino)-[2.2]-paracyclophane][(1S,2S)-(-)-1,2-diphenylethylenediamine]ruthenium(II),min.95%
Dichloro[(R)-(-)-4,12-bis(di(3,5-xylyl)phosphino)-[2.2]-paracyclophane][(1S,2S)-(-)-1,2-diphenylethylenediamine]ruthenium(II), min. 95%
Dichloro[(R)-(-)-4,12-bis(di(3,5-xylyl)phosphino)-[2.2]-paracyclophane][(1S,2S)
-(-)-1,2-diphenylethylenediamine]ruthenium (II), min. 95% | [Molecular Formula]
C62H66Cl2N2P2Ru | [MDL Number]
MFCD04974239 | [MOL File]
325150-57-0.mol | [Molecular Weight]
1073.12 |
| Questions And Answer | Back Directory | [Reaction]
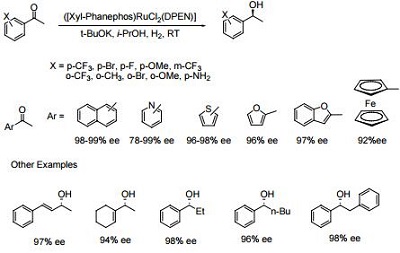
- The Noyori [(diphosphine) RuCl2 (diamine)] catalysts containing the chiral ligand Xylyl-Phanephos display exceptional activity and enantioselectivity in the asymmetric hydrogenation of a wide range of aromatic, heteroaromatic and α,β-unsaturated ketones.
- Reactions are performed under mild conditions at room temperature and typically at low H2 pressures of 2-10 bar. High substrate concentrations of up to 40% w/v are tolerated.
- Molar substrate/catalyst ratios of up to 100,000/1 are achieved with excellent reactivity and enantioselectivity using commercial grade substrates and solvents.
|
|
|