| Identification | Back Directory | [Name]
DIETHYL (CHLOROMETHYL)PHOSPHONATE | [CAS]
3167-63-3 | [Synonyms]
NSC 67753
3167-63-3
Diethyl (chloromethyl)
DIETHYL (CHLOROMETHYL)PHOSPHONATE
[DIETHYL-(CHLORMETHYL)]-PHOSPHONAT
Diethyl chloromethylphosphonate 97%
Diethyl (chloromethyl)phosphonate,98%
Diethyl(chloromethyl)phosphonate, 97 %
(chloromethyl)-phosphonicacidiethylester
(Chloromethyl)phosphonic acid diethyl ester | [EINECS(EC#)]
221-632-5 | [Molecular Formula]
C5H12ClO3P | [MDL Number]
MFCD00010189 | [MOL File]
3167-63-3.mol | [Molecular Weight]
186.57 |
| Chemical Properties | Back Directory | [Appearance]
Clear colorless to light yellow liquid | [Boiling point ]
109-110 °C10 mm Hg(lit.)
| [density ]
1.2 g/mL at 25 °C(lit.)
| [refractive index ]
n20/D 1.437(lit.)
| [Fp ]
188 °F
| [storage temp. ]
2-8°C
| [solubility ]
sol THF, ether, dichloromethane, chloroform | [form ]
Liquid | [color ]
Clear colorless to light yellow | [Specific Gravity]
1.2 | [BRN ]
1363361 | [EPA Substance Registry System]
Phosphonic acid, (chloromethyl)-, diethyl ester (3167-63-3) |
| Hazard Information | Back Directory | [Chemical Properties]
Clear colorless to light yellow liquid | [Uses]
Reactant involved in:
- Subsequent alkylation after nucleophilic substitution
- One-pot alkylation-boration of α-haloalkylphosphonates
- Synthesis of cyclpentane and pyrrolidine derivatives via regioselective insertion reactions
- Phosphorylation leading to P-containing cyclopropanes
- Wadsworth-Emmons reactions
- Ring expansion of zircnacycles via carbenoid insertion
| [Synthesis Reference(s)]
Tetrahedron Letters, 28, p. 3799, 1987 DOI: 10.1016/S0040-4039(00)96387-1 | [reaction suitability]
reaction type: C-C Bond Formation | [Synthesis]
Diethyl (chloromethyl)phosphonate is synthesized by a preparative scale of (EtO)2P(O)CH2Cl and other (1- chloroalkyl)phosphonates is relatively difficult. Ethanolysis of (chloromethyl)phosphonic dichloride is the most general and useful procedure (eq 1).
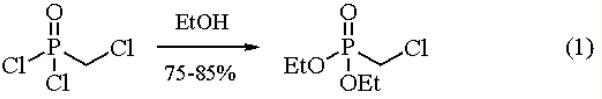
|
|
|