| Identification | Back Directory | [Name]
PGUQU 3F | [CAS]
303186-36-9 | [Synonyms]
PGUQU 3F
3-BB(F)B(F,F)XB(F,F)-F
4-[Difluoro(3,4,5-trifluorophenoxy)methyl]-2'3,5-Trifluoro-4...
Propyl-2,6,2 '- trifluorotriphenyl-difluoromethoxy-3,4,5-trifluorobenzene
4-[difluoro-(3,4,5-trifluorophenoxy)methyl]-2',3,5-trifluoro-4"-propyl-[1,1'
4-[Difluoro(3,4,5-trifluorophenoxy)methyl]-2'3,5-Trifluoro-4-n-propylterphenyl
4-[Difluoro(3,4,5-trifluorophenoxy)methyl]-2',3,5-trifluoro-4''-propyl-1,1':4',1''-terphenyl
1,1':4',1''-Terphenyl, 4-[difluoro(3,4,5-trifluorophenoxy)methyl]-2',3,5-trifluoro-4''-propyl- | [EINECS(EC#)]
619-490-8 | [Molecular Formula]
C28H18F8O | [MOL File]
303186-36-9.mol | [Molecular Weight]
522.43 |
| Chemical Properties | Back Directory | [Boiling point ]
531.9±50.0 °C(Predicted) | [density ]
1.335±0.06 g/cm3(Predicted) | [vapor pressure ]
0-0Pa at 20-50℃ | [form ]
Crystalline | [LogP]
6.5 at 25℃ and pH6.1 |
| Hazard Information | Back Directory | [Preparation]
The preparation of PGUQU 3F is as follows:1.00 g (2.90 mmol) of 5-[4-chloro-2,6-difluorophenyl)difluoromethoxy]-1,2,3-trifluorobenzene and 0.764 g (2.96 mmol, 1.02 equivalent amount) of 4'-propyl-3-fluoro-4-biphenylboronic acid were placed in a 50-ml SUS autoclave, after which 46.7 mg (0.05 equivalent amount) of TBAB, 1.10 mg (0.0008 equivalent amount) of 2-dicyclohexylphosphino-2',4',6'-triisopropylbiphenyl (purchased from Johnson Matthey Japan Inc.), and 4.94 mg (0.0004 equivalent amount) of 5% palladium carbon (50% moisture content, manufactured by N.E. Chemcat Corporation; E-Type) were placed in the autoclave. The autoclave was subsequently sealed, and the atmosphere therein was replaced with nitrogen. Thereafter, 3.0 ml of deaerated water and 1.0 ml of deaerated DMAC were added thereto, and 0.44 g (1.5 equivalent amount) of triethylamine was further added thereto, followed by stirring at the reaction liquid temperature of 95°C for 3 hours. The mixture was stirred at room temperature for 20 minutes, and 5.0 ml of toluene and 2.0 ml of water were added to the reaction mixture, followed by additional stirring for 15 minutes. The reaction yield was 99.4% (quantified by 19F NMR).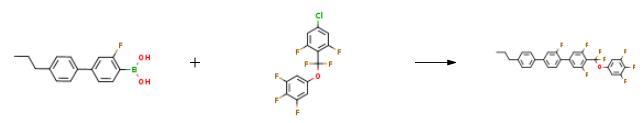
|
|
|