| Identification | Back Directory | [Name]
1-CYANOCYCLOBUTANECARBOXYLIC ACID ETHYL ESTER | [CAS]
28246-87-9 | [Synonyms]
ethyl 1-cyanocyclobutanecarboxylate
Ethyl 1-cyanocyclobutane-1-carboxylate
1-CYANOCYCLOBUTANECARBOXYLIC ACID ETHYL ESTER
1-Cyano-Cyclobutanecarboxylic Acid Ethyl Este
Cyclobutanecarboxylic acid, 1-cyano-, ethyl ester
(Z)-3-hydroxy-1-[5-(thiophen-2-ylmethyl)-2-furanyl]-3-(1H-1,2,4-triazol-5-yl)-2-propen-1-one | [Molecular Formula]
C8H11NO2 | [MDL Number]
MFCD04114273 | [MOL File]
28246-87-9.mol | [Molecular Weight]
153.18 |
| Hazard Information | Back Directory | [Uses]
1-CYANOCYCLOBUTANECARBOXYLIC ACID ETHYL ESTER can be used as organic synthesis intermediate and pharmaceutical intermediate, mainly used in laboratory research and development process and chemical and pharmaceutical production process. | [Synthesis]
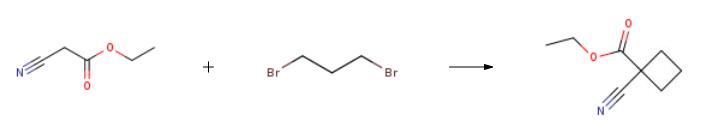
4-(9>-cyclopentyl-5'-methyl-6>-oxo-5>,6>,8>,9>- tetrahydrospiro[cyclobutane-l,7'-pyrimido[4,5-b][l,4]diazepine]-2'-ylamino)-3- methoxybenzoic acid; [0521] Ethyl 1-cyanocyclobutanecarboxylate; To a solution of sodium ethoxide (21wt% in EtOH, 5.79 rnL, 15.5 mmol) in EtOH (25 mL), was added ethyl cyanoacetate(1.12 mL, 10.5 mmol), followed shortly thereafter by 1,1-dibromopropane (1.12 mL, 10 mmol). The reaction mixture was refluxed for 3 hrs. It was then concentrated, and diluted to ethyl acetate. The organic layer was washed with NaHCCh, brine, water, dried over Na2SO4 and concentrated in vacuo to obtain ethyl 1-cyanocyclobutanecarboxylate (1.17 g, 74% yield) as a red liquid. It was used directly for next step reaction. |
|
|