| Identification | Back Directory | [Name]
1-Ethylcyclohexyl methacrylate | [CAS]
274248-09-8 | [Synonyms]
1-Ethylcyclohexyl methacrylate
Methacrylic Acid 1-Ethylcyclohexyl Ester
(1-ethylcyclohexyl) 2-methylprop-2-enoate
1-Ethylcyclohexyl methacrylate 274248-09-8
2-Propenoic acid, 2-methyl-, 1-ethylcyclohexyl ester
1-Ethylcyclohexyl Methacrylate (stabilized with MEHQ)
EtCHMA 1-Ethylcyclohexyl methacrylate ArF monomers
EtCHMA 2-Propenoic acid,2-methyl-,1-ethylcyclohexyl ester | [Molecular Formula]
C12H20O2 | [MOL File]
274248-09-8.mol | [Molecular Weight]
196.286 |
| Hazard Information | Back Directory | [Description]
1-Ethylcyclohexyl methacrylate (ECHM) is a type of methacrylate monomer,
a monofunctional acrylate monomer that is used in the synthesis of
polymers and other materials. It is a colorless liquid with a faint odor
and a low volatility. ECHM is used in a variety of industries,
including the production of adhesives, coatings, elastomers, and
plastics. It is also used in the production of polymers and other
materials used in medical and industrial applications. | [Chemical Properties]
Colorless to Light yellow clear liquid | [Uses]
1-Ethylcyclohexyl methacrylate is used as Photoresist monomer. | [Application]
1-Ethylcyclohexyl methacrylate can be used as analog semiconductor, light emitting diode LED and solar photovoltaic. | [Preparation]
The preparation of 1-Ethylcyclohexyl methacrylate is as follows:Weigh 34.3g (1.4mol) of magnesium chips,10 g (0.04 mol) of cesium chloride and 100 g of diethyl ether were placed in a 2 L three-necked flask and protected by dry nitrogen. Weigh 222.4g (2.04mol) of ethyl bromide,100g (1.02mol)After cyclohexanone is uniformly mixed with 500 g of diethyl ether, a preliminary solution I is formed and placed in a constant pressure dropping funnel.The internal temperature is 32 °C. Add 5 mL of the preliminary solution I to the magnesium chips, and after the reaction is initiated, a large amount of heat is released.The internal temperature rises to 60 ° C,The solution turned grey and white smoke appeared. When the internal temperature drops to 40 ° C, the preliminary solution I is continuously added dropwise and stirring is started. Control the internal temperature <65 ° C, lh drops,After maintaining the temperature at 40 ° C for 2 h. Add 8.96g(0.08 mol) potassium t-butoxide, 10 g phenothiazine.188.6 g (1.2 mol) of methacrylic anhydride was weighed and mixed with 100 g of diethyl ether to form a preliminary liquid II, which was placed in a constant pressure dropping funnel. When the internal temperature of the reaction system is 40 ° C,Start adding the preliminary solution II, and with the addition of the mixed solution,The reaction was slowly exothermic and the internal temperature was controlled to drop below 65 °C. After 30 minutes,After stirring at 50 ° C for 3 h, the reaction was stopped, and after cooling to 25 ° C,The reaction solution was poured into 1 L of ice water and stirred for 40 min. Liquid separation,The aqueous phase was extracted with 150. 0 g x 2 of diethyl ether.The organic phases were combined, washed with 50.0 g of 2 NaOH aqueous solution, and washed with 200 g×2 pure water.Drying with 100. 0 g of anhydrous sodium sulfate, suction filtration,Distillation under reduced pressure, collecting fractions at 60 Pa (60-80 ° C),180.6 g of a colorless transparent liquid was obtained, and the nuclear magnetic spectrum confirmed that the structure was correct.The purity of the GC was 99.52%, and the yield was 90.3%.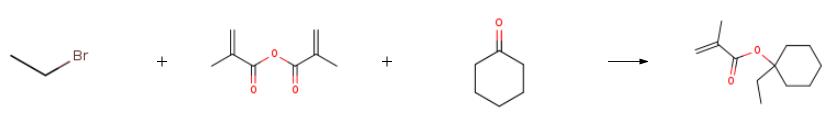
|
|
|