| Identification | Back Directory | [Name]
CHLOROTRIS(TRIPHENYLPHOSPHINE)COBALT(I) | [CAS]
26305-75-9 | [Synonyms]
CoCl(Ph3P)3
Cobalt,triphenylphosphane,chloride
Chlorotris(triphenylphosphine)cobalt
Tris(triphenylphosphine)cobalt chloride
CHLOROTRIS(TRIPHENYLPHOSPHINE)COBALT(I)
cobalt(I) tris(triphenylphosphine) chloride
Chlorotris(triphenylphosphine)cobalt(I)
Chlorotris(triphenylphosphine)cobalt(I),Min.98%
Chlorotris(triphenylphosphine)cobalt(I), min. 98%
Chlorotris(triphenylphosphine)cobalt(I),98% (PPh3)3CoCl | [Molecular Formula]
C54H45ClCoP3 | [MDL Number]
MFCD00015864 | [MOL File]
26305-75-9.mol | [Molecular Weight]
881.24 |
| Chemical Properties | Back Directory | [Melting point ]
135-139 °C (dec.)(lit.)
| [storage temp. ]
Inert atmosphere,Room Temperature | [solubility ]
sol benzene and CH2Cl2; insol EtOH. | [form ]
Powder | [color ]
brown | [Water Solubility ]
Insoluble in water. | [Sensitive ]
Air & Moisture Sensitive |
| Hazard Information | Back Directory | [Description]
Chlorotris(triphenylphosphine)cobalt is a monovalent cobalt complex; homogeneous catalyst for hydrogenation and hydrodimerization of
alkenes; strong nucleophile, reacting with alkyl acyl halides to afford coupling products; useful for
the preparation of organocobalt complexes. | [Uses]
A stoichiometric reducing agent employed in the radical dimerization of halogenated organic molecules. | [Uses]
A stoichiometric reducing agent that is used in the radical dimerization of halogenated organic molecules. | [Preparation]
Preparative Methods of Chlorotris(triphenylphosphine)cobalt: a solution of Cobalt(II) Chloride hexahydrate (0.6 g, 2.5 mmol) and
Triphenylphosphine (2.0 g, 7.6 mmol) in EtOH (70 mL) was treated under N2 and stirring with a
solution of Sodium Borohydride (0.08 g, 2.1 mmol) in EtOH at 30-40 °C; the resulting brown-green
precipitate was washed several times with EtOH and water and dried (yield 92%);[1] reductions with
powdered Zn, and electrolysis, are equally effective methods; corresponding Br and I complexes are
prepared similarly from CoBr2 and CoI2. | [storage]
Chlorotris(triphenylphosphine)cobalt is fairly stable in air; it has been stored under
N2 atmosphere over several months at rt. | [References]
1. (a) Aresta, M.; Rossi, M.; Sacco, A. ICA 1969, 3, 227. (b) Holah, D. G.; Hughes, A. N.; Hui, B. C.; Kan, C.
T. CJC 1978, 56, 814. |
| Questions And Answer | Back Directory | [Reaction]
- Cobalt(I)-catalyzed, steroselective olefination of alkylzinc reagents with aryl aldehydes.
- Reagent for the reductive radical dimerization of 3-haloindoline derivatives.
- Cobalt-catalyzed, asymmetric addition of silylacetylene to 1,1-disubstituted allenes.
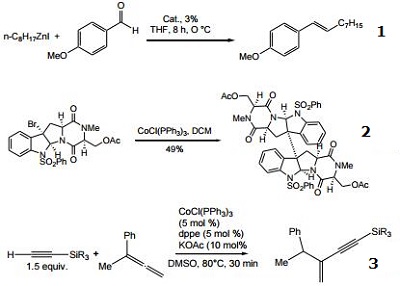
|
|
| Company Name: |
Alfa Aesar
|
| Tel: |
400-6106006 |
| Website: |
http://chemicals.thermofisher.cn |
|