| Identification | Back Directory | [Name]
(R)-(+)-5,5'-Bis(diphenylphosphino)-4,4'-bi-1,3-benzodioxole,min.98%(R)-SEGPHOS | [CAS]
244261-66-3 | [Synonyms]
(R)-SEGPHOS(R)
(R)-(+)-SEGPHOS
(R)-SEGPHOS(R) >=94%
(R)-(+)-SEGPHOS(regR)
(R)-(+)-SEGPHOS®
(R)-5,5'-Bis(diphenylphosphino)-4,4'-bibenzo[d][1,3]dioxole
(R)-(+)-5,5'-Bis(diphenylphosphino)-4,4'-bi-1,3-benzodioxole
[(4R)-[4,4'-Bi-1,3-benzodioxole]-5,5'-diyl]bis[diphenylphosphine]
(R)-(+)-5,5'-Bis(diphenylphosphino)-4,4'-bi-1,3-benzodioxole, min. 98%
Phosphine,1,1'-[(4R)-[4,4'-bi-1,3-benzodioxole]-5,5'-diyl]bis[1,1-diphenyl-
(R)-(+)-5,5'-Bis(diphenylphosphino)-4,4'-bi-1,3-benzodioxole,(R)-(+)-SEGPHOS
(R)-(+)-5,5'-Bis(diphenylphosphino)-4,4'-bi-1,3-benzodioxole,min.98%(R)-SEGPHOS
(R)-(+)-5,5'-Bis(diphenylphosphino)-4,4'-bi-1,3-benzodioxole, min. 98% (R)-(+)-SEGPHOS(R)
(R)-(+)-5,5μ-Bis(diphenylphosphino)-4,4μ-bi-1,3-benzodioxole, [4(R)-(4,4μ-bi-1,3-benzodioxole)-5,5μ-diyl]bis[diphenylphosphine] | [Molecular Formula]
C38H28O4P2 | [MDL Number]
MFCD09753005 | [MOL File]
244261-66-3.mol | [Molecular Weight]
610.59 |
| Chemical Properties | Back Directory | [Melting point ]
168-172 °C | [Boiling point ]
715.4±60.0 °C(Predicted) | [storage temp. ]
Inert atmosphere,Room Temperature | [form ]
Powder | [color ]
off-white | [optical activity]
[α]20/D +11°, c = 0.5 in chloroform |
| Questions And Answer | Back Directory | [Reactions]
- Biaryl bisphosphine ligand with narrow dihedral angle. The SEGPHOS® ligand has been applied to a variety of metal catalyzed reactions. In many cases, yields and enantioselectivities, exceed results obtained earlier using BINAP.
- As ruthenium complex, SEGPHOS® generally gives higher levels of chiral induction in asymmetric hydrogenations of α,β,and γ-functionalized ketones.
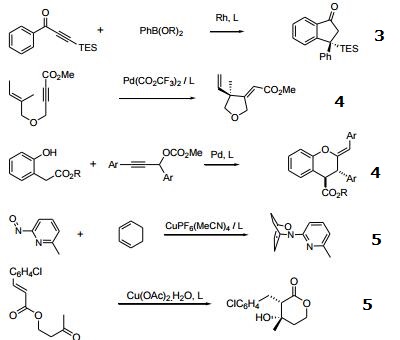
- Used in Rh-catalyzed transformations such as: (a) 1,4-addition of boronic acids to coumarins, (b) addition of titanium reagents to imines,(c) cotrimerization of alkenes and acetylenes,10 (d)double [2+2+2] cycloaddition,11 (e) indanone formation.
- Used in Pd-catalyzed transformations such as: (a) cycloaddition of 1,6-enyne,(b) arylative cyclization of allenyl aldehydes with boronic acids,13 (c) synthesis of chromans.
- Used in Cu-catalyzed transformations such as: (a) nitroso Diels-Alder,(b) reductive aldol condensation,(c) conjugate reduction of unsaturated sulfones,and phophonates.
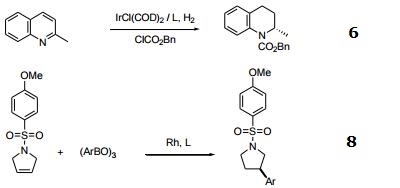
- Iridium-catalyzed asymmetric hydrogenation of quinolines activated by chloroformates.
- Iridium-catalyzed asymmetric transfer hydrogenation used in polyketide construction.
- Rhodium-catalyzed asymmetric hydroarylation of 3-pyrrolines.
- Palladium-catalyzed regio- and enantioselective dearomatization.
|
| Hazard Information | Back Directory | [Uses]
(R)-(+)-SEGPHOS? is a chelating ligand used to prepare coordination complex catalysts, such as its use in Pd catalysts for the enantioselective addition of diesters to 3,3-difluoropropenes, and in the Ni-based catalyst for the asymmetric propargylic amination of propargylic carbonates with an internal alkyne group. |
|
|