| Identification | Back Directory | [Name]
Dichloro{(S)-(-)-2,2'-bis[di(3,5-xylyl)phosphino]-1,1'-binaphthyl}[(2S)-(+)-1,1-bis(4-methoxyphenyl)-3-methyl-1,2-butanediamine]ruthenium(II) | [CAS]
220114-01-2 | [Synonyms]
RuCl2[(S)-xylbinap][(S)-daipen]
RuCl2[(S)-(DM-BINAP)][(S)-DAIPEN]
Dichloro{(S)-(-)-2,2'-bis[di(3,5-xylyl)phosphino]-1,1'-binaphthyl}[(2S)-(+)-1,1-bis(4-methoxyphenyl)-3-methyl-1,2-butanediamine]ruthenium(II)
Dichloro{(S)-(-)-2,2'-bis[di(3,5-xylyl)phosphino]-1,1'-binaphthyl}[(2S)-(+)-1,1-bis(4-methoxyphenyl)-3-methyl-1,2-butanediamine]ruthenium(II) RuCl2[(S)-xylbinap][(S)-daipen] | [Molecular Formula]
C71H74Cl2N2O2P2Ru | [MDL Number]
MFCD09753027 | [MOL File]
220114-01-2.mol |
| Questions And Answer | Back Directory | [Reaction]
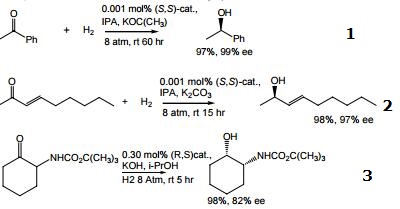
Highly active catalyst for hydrogenation of simple ketones giving high enantioselectivity when sterically unsymmetrical ketones such as acetophenone, heteroaryl ketones, benzophenones, cyclopropyl ketones, and cyclohexyl ketones are substrates. Ee's are enhanced with XylBINAP relative to BINAP. The otherwise poorly bonded ketone is held in the transition state by hydrogen bonding to the protic bidentate amine.
Carbonyl groups are selectively reduced even when olefins exist in the same molecule.
In the presence of strong base, and catalyst, simple ketones, having substituents at the α-position, may be induced to undergo dynamic kinectic resolution during their hydrogenation to produce two chiral carbon centers in high yield.
|
| Hazard Information | Back Directory | [Uses]
Catalyst for:
- Preparation of cyclometalated ruthenium 1,1-dianisyl-2-isopropyl-1,2-ethylenediamine complexes via asymmetric hydrogenation of ketones
- Enantioselective hydrogenation of an α-alkoxy substituted ketone with chiral ruthenium (phosphinoferrocenyl)oxazoline complexes
- Nonclassical asymmeteric hydrogen transfer between alcohols and carbonyl compounds
- Asymmetric hydrogenation of amino and heteroaromatic ketones
- Pharmacologically active substituted oxazolidinone used as NPC1L1 ligand for inhibition of cholesterol absorption
|
|
| Company Name: |
Energy Chemical
|
| Tel: |
021-021-58432009 400-005-6266 |
| Website: |
http://www.energy-chemical.com |
| Company Name: |
Sigma-Aldrich
|
| Tel: |
021-61415566 800-8193336 |
| Website: |
https://www.sigmaaldrich.cn |
|