| Identification | Back Directory | [Name]
(+)-1,2-BIS((2S,5S)-2,5-DIETHYLPHOSPHOLANO)BENZENE(CYCLOOCTADIENE)RHODIUM(L)TETRAFLUOROBORATE | [CAS]
213343-64-7 | [Synonyms]
(+)-Bisdiethylphospholanobenzenecyclooctadienerhod
(+)-1,2-Bis((2S,5S)-2,5-diethylphospholano)benzene(cyclooctadiene)rhodium(l)tetrafluroborate
(+)-1,2-Bis((2S,5S)-2,5-diethylphospholano)benzene(cyclooctadiene)rhodium(I) tetrafluroborate
(+)-1,2-BIS((2S,5S)-2,5-DIETHYLPHOSPHOLANO)BENZENE(CYCLOOCTADIENE)RHODIUM(L)TETRAFLUOROBORATE
1,2-Bis[(2S,5S)-2,5-diethylphospholano]benzene(1,5-cyclooctadiene)rhodium(Ⅰ) tetrafluoroborate
(+)-1,2-BIS((2S,5S)-2,5-DIETHYLPHOSPHOLANO)BENZENE(CYCLOOCTADIENE)RHODIUM (I) TETRAFLUOROBORATE
1,2-Bis[(2S,5S)-2,5-diethylphospholano]benzene(1,5-cyclooctadiene)rhodium(I) tetrafluoroborate
(+)-1,2-Bis((2S,5S)-2,5-diethylphospholano)benzene(cyclooctadiene)rhodium(I)tetrafluoroborate,98+%(S,S)-Et-DUPHOS-Rh
(+)-1,2-Bis((2S,5S)-2,5-diethylphospholano)benzene(1,5-cyclooctadiene)rhodium(I) tetrafluoroborate, 98+% (S,S)-Et-DUPHOS-Rh | [Molecular Formula]
C30H48BF4P2Rh- | [MDL Number]
MFCD03412156 | [MOL File]
213343-64-7.mol | [Molecular Weight]
660.36 |
| Hazard Information | Back Directory | [Chemical Properties]
Dark orange-red solid | [Uses]
(S,S)-Et-DUPHOS-Rh is a catalyst in the asymmetric preparation of B precursor of cryptophycin, and in the synthesis of constrained phenylalanine analogs. | [Uses]
DuPhos and BPE Ligands: Highly Efficient Privileged Ligands
Catalyst for:
- Preparation of a key unit B precursor of cryptophycins via Horner-Wadsworth-Emmons reaction and asymmetric hydrogenation
- Preparation of constrained phenylalanine analogs via Heck reaction followed by asymmetric hydrogenation and cyclization steps
- Enantioselective preparation of N-benzyloxy-β-amino acid Me esters by hydrogenation of α-(benzyloxyaminomethyl)acrylate stereoisomers
- Preparation of fluoro amino acids as synthons for potent macrocyclic HCV NS3 protease inhibitors
- Stereoselective preparation of triply isotope-labeled Ser, Cys, and Ala
- Preparation of β-amino acids from asymmetric hydrogenation of α-(aminomethyl)acrylates
- Asymmetric hydrogenation of a-primary and secondary amino ketones and asymmetric synthesis of (-)-arbutamine and (-)-denopamine
|
| Questions And Answer | Back Directory | [Reactions]
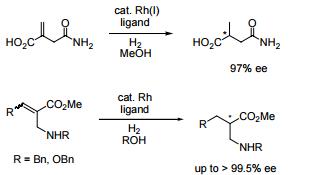
- Ligand used in asymmetric hydrogenation of 2-methylenesuccinamic acid.
- Ligand used for the Rh-catalyzed asymmetric hydrogenation of α-aminomethylacrylates.
|
|
| Company Name: |
Alfa Aesar
|
| Tel: |
400-6106006 |
| Website: |
http://chemicals.thermofisher.cn |
| Company Name: |
Energy Chemical
|
| Tel: |
021-021-58432009 400-005-6266 |
| Website: |
http://www.energy-chemical.com |
| Company Name: |
Sigma-Aldrich
|
| Tel: |
021-61415566 800-8193336 |
| Website: |
https://www.sigmaaldrich.cn |
| Company Name: |
Alfa Chemistry
|
| Tel: |
1-516-6625404 |
| Website: |
https://www.alfa-chemistry.com |
|