| Identification | Back Directory | [Name]
METHYL 4-IODOSALICYLATE | [CAS]
18179-39-0 | [Synonyms]
Methyl4-Iodosilicylate
Methyl4-Iodosalicylate>
Methyl 4-iodosalicylate 97%
4-Iodosalicylic Acid Methyl Ester
2-Hydroxy-4-iodobenzoic Acid Methyl Ester
Benzoic acid, 2-hydroxy-4-iodo-, methyl ester
Methyl 4-iodosalicylate, 5-Iodo-2-(methoxycarbonyl)phenol
Methyl 2-Hydroxy-4-iodobenzoate
4-Iodosalicylic Acid Methyl Ester
2-Hydroxy-4-iodobenzoic Acid Methyl Ester | [Molecular Formula]
C7H7IO2Si | [MDL Number]
MFCD06797864 | [MOL File]
18179-39-0.mol | [Molecular Weight]
278.119 |
| Chemical Properties | Back Directory | [Melting point ]
69-73 °C
| [Boiling point ]
309.7±32.0 °C(Predicted) | [density ]
1.880±0.06 g/cm3(Predicted) | [storage temp. ]
Keep in dark place,Inert atmosphere,Room temperature | [form ]
powder to crystal | [pka]
8.91±0.10(Predicted) | [color ]
White to Gray to Brown | [Sensitive ]
Light Sensitive | [InChIKey]
WUFUURSWOJROKY-UHFFFAOYSA-N |
| Safety Data | Back Directory | [Hazard Codes ]
T | [Risk Statements ]
25 | [Safety Statements ]
45 | [RIDADR ]
UN 2811 6.1/PG 3
| [WGK Germany ]
3
| [F ]
8 | [Hazard Note ]
Toxic | [PackingGroup ]
III | [HS Code ]
2918290090 |
| Questions And Answer | Back Directory | [Structure]
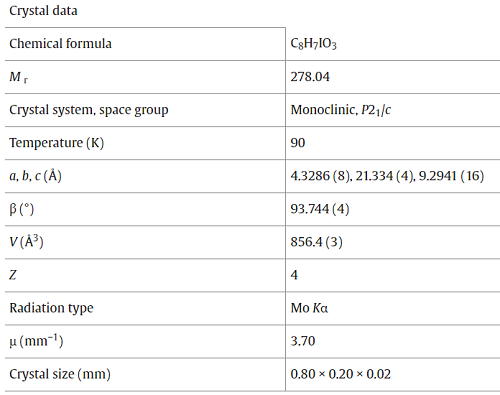
2-hy-droxy-4-iodo-benzoic acid methyl ester (methyl 4-iodo-salicylate, C8H7IO3) allows for an effective way of incorporating the methyl salicylates within larger organic mol-ecules, using such methodologies as McClure protocols, which take advantage of the iodine atom at the 4-position of the aromatic ring for the formation of carbon-carbon bonds. The iodine atom is also capable of forming supra-molecular synthons, which may be useful for crystal engineering. At 90?K, the methyl 4-iodo-salicylat displays monoclinic (P21/c) symmetry with one mol-ecule in the asymmetric unit. Inter-molecular hydrogen bonding inter-actions occur between the hy-droxy groups of one mol-ecule and the carbonyl oxygen atom of the methyl ester of an adjacent mol-ecule to form a centrosymmetric dimeric pair with H?O = 2.53?(4)??. An O3—H3?O2 intra-molecular hydrogen bond also exists with an H?O distance of 2.05?(4)??. The C5?C8 [3.326?(3)??] and O3?H1C (2.51??) inter-actions provide only short contacts between the stacks of offset (102) parallel sheets, which make up the crystal. These sheets, in turn, contain the inversion-generated hydrogen-bonded dimers. The non-hydrogen atoms of the mol-ecule are essentially coplanar with no displacement from the mean mol-ecular plane greater than 0.132??.
 |
|
|