| Identification | Back Directory | [Name]
1-Methylcyclopentyl methacrylate | [CAS]
178889-45-7 | [Synonyms]
1-Methylcyclopentyl methacrylate
MCPMA 1-Methylcyclopentyl methacrylate
Methacrylic Acid 1-Methylcyclopentyl Ester
1-Methylcyclopentyl methacrylate 178889-45-7
1-Methylcyclopentyl methacrylate MCPMA ArF monomers
2-Propenoic acid, 2-methyl-, 1-methylcyclopentyl ester
1-Methylcyclopentyl Methacrylate (stabilized with MEHQ)
2-Propenoic acid, 2-methyl-, 1-methylcyclopentyl ester 178889-45-7 | [Molecular Formula]
C10H16O2 | [MDL Number]
MFCD31715003 | [MOL File]
178889-45-7.mol | [Molecular Weight]
168.233 |
| Chemical Properties | Back Directory | [Boiling point ]
205.2±9.0 °C(Predicted) | [density ]
0.96±0.1 g/cm3(Predicted) | [Fp ]
74 °C | [storage temp. ]
Room temperature ( recommended cool, dark place below 15°C) | [form ]
clear liquid | [color ]
Colorless to Light yellow | [Specific Gravity]
0.95 | [InChI]
InChI=1S/C10H16O2/c1-8(2)9(11)12-10(3)6-4-5-7-10/h1,4-7H2,2-3H3 | [InChIKey]
VSYDNHCEDWYFBX-UHFFFAOYSA-N | [SMILES]
C(OC1(C)CCCC1)(=O)C(C)=C |
| Hazard Information | Back Directory | [Description]
1-Methylcyclopentyl methacrylate, also known as Methacrylic Acid 1-Methylcyclopentyl Ester, is a organic compound. It is commonly used as the Photoresist monomer. Photoresist (photo reactive materials) is a light-sensitive material used in several processes, such as photolithography and photoengraving, to form a patterned coating on a surface. Lithographic techniques such as deep X-ray lithography (DXRL), X-ray lithography (XRL), deep ultraviolet lithography (DUVL) and ultraviolet lithography (UVL) rely on this material[1]. | [Chemical Properties]
Colorless to Light yellow clear liquid | [Uses]
1-Methylcyclopentyl methacrylate is used as Photoresist monomer. | [Application]
1-Methylcyclopentyl methacrylate can be applied in analog semiconductor, light-emitting diode LED, solar photovoltaic and other fields. | [Preparation]
The preparation of 1-Methylcyclopentyl methacrylate is as follows:Add 1-methylcyclopentanol (730g, 7.29mol), dichloromethane (5100g), triethylamine (1819g, 17.98mol), 4-dimethylaminopyridine (8.9g, 0.0728mol) to the 10L four-necked flask Stirring and cooling with phenothiazine (7.3g) were started, and methacryloyl chloride (1143g, 10.93mol) was added dropwise at a temperature of 0 to 5℃., and the temperature was kept for 2h after the dropwise addition. (raw material≦2.0%).Water (2000g) was added to quench, and the temperature was controlled not to exceed 20℃. The organic phase was washed twice with 5% hydrochloric acid (2000g*2), twice with 5% aqueous sodium hydroxide solution (2000g*2), and twice with water (2000g*2), the organic phase was dried by adding anhydrous sodium sulfate, and spun. The solvent was evaporated and concentrated to obtain 1268g of crude product. The crude product was distilled under reduced pressure (negative pressure: 1mmHg column, external temperature: 60-70℃., main fraction: 35-40℃.) to obtain 921g of product.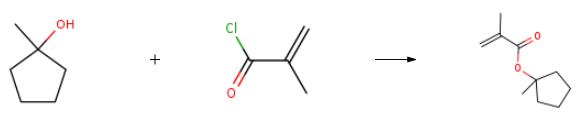
| [Solubility in organics]
Very soluble in N,N-Dimethylformamide. Soluble in methanol. Sparingly
soluble inglacial acetic acid. Very slightly soluble inchloroform. Practically insoluble in water. | [References]
[1] Vlnieska V, et al. Epoxy Resins for Negative Tone Photoresists. Polymers, 2019; 11: 1457. |
|
|