| Identification | Back Directory | [Name]
(R)-MONOPHOS | [CAS]
157488-65-8 | [Synonyms]
(R)-MONOPHOS
(S)-MONOPHOS
97% (R)-MONOPHOS
3,4-a']dinaphthalen
3,4-a']dinaphthalen-4-yl)Morpholine
3,4-a']dinaphthalen-4-yl)piperidine
3,4-a']dinaphthalen-4-yl)dipropylaMine
3,4-a']dinaphthalen-4-yl)dimethylamine
(R)-(-)-(3,5-Dioxa-4-phospha-cyclohepta[2,1-a
3,4-a']dinaphthalen-4-yl)[(1S)-1-phenylethyl]-aMine
3,4-a']dinaphthalen-4-yl)dimethylamine,97% (R)-MONOPHOS
3,4-a']dinaphthalen-4-yl)dimethylamine,min.97%(R)-MONOPHOS
-N,N-Dimethyldinaphtho[2,1-d:1',2'-f][1,3,2]dioxaphosphepin-4-amine
R-8,9,10,11,12,13,14,15,Octahydro-3,5-dioxa-4-phosphacyclohepta[2,1-a
(R)-N,N-DiMethyldinaphtho[2,1-d:1',2'-f][1,3,2]dioxaphosphepin-4-aMine
(11bR)-N,N-Dimethyl-dinaphtho[2,1-d:1',2'-f][1,3,2]dioxaphosphepin-4-amine
(R)-(-)-[4-N,N-DIMETHYLAMINO]DINAPHTHO[2,1-D:1',2'-F][1,3,2]DIOXAPHOSPHEPINE
(S)-(+)-[4-N,N-DIMETHYLAMINO]DINAPHTHO[2,1-D:1',2'-F][1,3,2]DIOXAPHOSPHEPINE
(11bR)-N,N-Dimethyl-dinaphtho[2,1-d:1',2'-f][1,3,2]
dioxaphosphepin-4-amine,99%e.e.
(R)-(-)-(3,5-DIOXA-4-PHOSPHA-CYCLOHEPTA[2,1-A:3,4-A']DI-NAPHTHALEN-4-YL)DIMETHYLAMINE
(S)-(+)-(3,5-DIOXA-4-PHOSPHA-CYCLOHEPTA[2,1-A:3,4-A']DI-NAPHTHALEN-4-YL)DIMETHYLAMINE
(R)-(-)-(3,5-Dioxa-4-phosphacyclohepta[2,1-a:3,4-a']dinaphthalen-4-yl)diMethylaMine 97%
(R)-(-)-[4-N,N-Dimethylamino]dinaphtho[2.1-d:1'.2'-f][1.3.2]dioxaphosphepine(R)-monophos]
(R)-()-(3,5-Dioxa-4-phospha-cyclohepta[2,1-a:3,4-a′]di-naphthalen-4-yl)dimethylamine,(R)-Monophos
(R)-(-)-(3,5-Dioxa-4-phospha-cyclohepta[2,1-a:3,4-a']dinaphthalen-4-yl)dimethylamine, min. 97% (R)-MONOPHOS | [Molecular Formula]
C22H18NO2P | [MDL Number]
MFCD03426988 | [MOL File]
157488-65-8.mol | [Molecular Weight]
359.36 |
| Chemical Properties | Back Directory | [Melting point ]
190 °C (dec.)
| [alpha ]
-583° (c 0.06, CHCl3) | [Boiling point ]
548.7±33.0 °C(Predicted) | [storage temp. ]
Inert atmosphere,Room Temperature | [form ]
crystal | [pka]
1.22±0.20(Predicted) | [color ]
white | [Sensitive ]
air sensitive | [InChIKey]
QCHAVHXSBZARBO-UHFFFAOYSA-N |
| Questions And Answer | Back Directory | [Reaction]
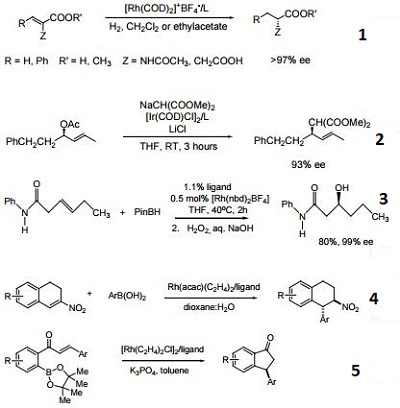
- Ligand used in the enantioselective, rhodium-catalyzed hydrogenation of substituted olefins, such as N-acetyldihydroamino acids, enamides, and unsaturated acids.
- Ligand used in the enantioselective, iridium-catalyzed allylic substitution of allyl acetates containing only a single substituent in the 1 or 3 position.
- Ligand use in the rhodium-catalyzed, amide directed, asymmetric hydroboration reaction.
- Ligand used in asymmetric conjugate addition of aryl boronic acids to dihydronitronaphthalenes.
- Ligand used in the rhodium-catalyzed asymmetric intramolecular 1,4 addition.
|
|
|