| Identification | Back Directory | [Name]
DIIRON NONACARBONYL | [CAS]
15321-51-4 | [Synonyms]
Fe-Fe
Fe2(CO)9
Diiroonacarbonyl
IRON NANOCARBONYL
IRON NONACARBONYL
NONACARBONYLDIIRON
Iron enneacarbonyl
Nonocarbonyldiiron
DIIRON NONACARBONYL
Diiron ennecarbonyl
Enneacarbonyldiiron
Diiron enneacarbonyl
Ironnonacarbonyl,99%
Iron, nonacarbonyldi-
Diironnonacarbonyl 98%
Diiron nonacarbonyl,98%
Diiron nonacarbonyl,99%
Iron carbonyl (Fe2(CO)9)
Carbon Monooxide - iron (9:2)
tri-μ-Carbonylhexacarbonyldi-Iron
Tri-mu-carbonylhexacarbonyldiiron
Iron,tri-μ-Carbonylhexacarbonyldi-
Iron, tri-mu-carbonylhexacarbonyldi-
tri-mu-carbonylhexacarbonyldi-iro(fe-fe)
Tri-mu-carbonylhexacarbonyldiiron, (Fe-Fe)
Nonacarbonyldiiron (Fe2(CO)9), nonacarbonyldi-
Iron, tri-.mu.-carbonylhexacarbonyldi-, (Fe-Fe) | [EINECS(EC#)]
239-359-5 | [Molecular Formula]
C9Fe2O9 | [MDL Number]
MFCD00151465 | [MOL File]
15321-51-4.mol | [Molecular Weight]
363.78 |
| Hazard Information | Back Directory | [Chemical Properties]
yellow to orange platelets | [Description]
This carbonyl is obtained as dark yellow platelets by exposing solutions of the pentacarbonyl
in organic solvents to sunlight or ultraviolet irradiation. | [Uses]
It is an important reagent in?organometallic chemistry?and of occasional use in?organic synthesis. | [Purification Methods]
Wash it with EtOH and Et2O, then dry it in air. It sublimes at 35o at high vacuum. It forms dark yellow plates which are stable for several days when kept in small amounts. Large amounts, especially when placed in a desiccator, spontaneously ignite in a period of one day. It decomposes in moist air. It is insoluble in hydrocarbon solvents but forms complexes with several organic compounds. [Sheline & Pitzer J Am Chem Soc 72 1107 1950, Speyer & Wolf Chem Ber 60 1424 1927.] TOXIC. | [Structure and conformation]
Iron enneacarbonyl decomposes at 100°, but can be sublimed at 35°C in a high vacuum.
It is insoluble in aliphatic hydrocarbons and reacts with many organic solvents to form
mainly the pentacarbonyl or its substituted derivatives. These properties have considerably
hindered structural studies ; the infrared spectrum, for example, can only be measured in
the solid. The X-ray crystal structure determination shows it to have the structure shown
in Fig. 8 in which six carbon monoxide molecules are arranged around each iron atom in a
slightly distorted octahedral array. The Fe-Fe distance is 2.46 埃, whilst the Fe-C distances
are all around 1.85 埃. The Mossbauer spectrum also suggests that the iron atoms are in
an octahedral environment. However, since the compound is diamagnetic it must contain
an Fe-Fe bond as well as the three bridging carbonyl groups.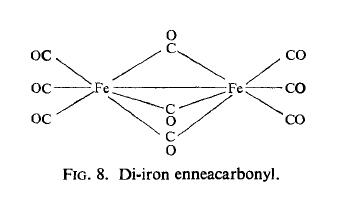 |
|
| Company Name: |
Alfa Aesar
|
| Tel: |
400-6106006 |
| Website: |
http://chemicals.thermofisher.cn |
|