| Identification | Back Directory | [Name]
1,3,4,6,7,9,9b-heptaazaphenalene-2,5,8-triamine | [CAS]
1502-47-2 | [Synonyms]
Meleme
2,4,6-Triazine symmheptazine
1,3,4,6,7,9,9b-Heptaazaphenalen-2,5,8-triamin
1,3,4,6,7,9,9b-heptaazaphenalene-2,5,8-triamine
2,5,8-Triamino-1,3,4,6,7,9,9b-heptaazaphenalene
1,3,3a1,4,6,7,9-Heptaazaphenalene-2,5,8-triamine
1,3,4,6,7,9,9b-Heptaaza-9bH-phenalene-2,5,8-triamine
2,5,8-Triamino-1,3,4,6,7,9,9b-heptaaza-9bH-phenalene | [EINECS(EC#)]
216-122-4 | [Molecular Formula]
C6H6N10 | [MDL Number]
MFCD00767872 | [MOL File]
1502-47-2.mol | [Molecular Weight]
218.18 |
| Chemical Properties | Back Directory | [Boiling point ]
348.86°C (rough estimate) | [density ]
1.6205 (rough estimate) | [vapor pressure ]
0Pa at 20℃ | [refractive index ]
1.9320 (estimate) | [pka]
-0.74±0.20(Predicted) | [Water Solubility ]
930μg/L at 20℃ | [LogP]
-0.2 at 20℃ |
| Hazard Information | Back Directory | [Uses]
1,3,4,6,7,9,9b-heptaazaphenalene-2,5,8-triamine can be used as organic synthesis intermediate and pharmaceutical intermediate, mainly used in laboratory research and development process and chemical production process. | [Definition]
ChEBI: Melem is a heptaazaphenalene. | [Flammability and Explosibility]
Notclassified | [Synthesis]
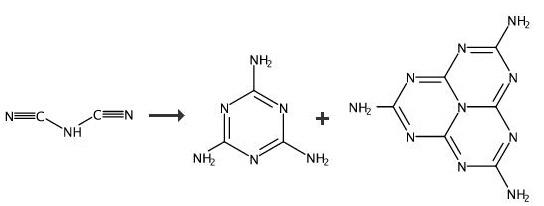
Melem 4e was synthesized by heating cyanamide 5 (Fluka, ≥98%) or ammonium dicyanamide 6 (for preparation, see ref 24) or dicyandiamide 7 (Avocado, 99%) or melamine 1a (Fluka, purum, ≥99% (NT)). The commercial products were used as purchased: 80 mg of starting material (1.90 mmol of 5, 0.95 mmol of 6, 0.95 mmol of 7, or 0.63 mmol of 1a, respectively) was filled into a glass ampule (outer diameter, 16 mm; inner diameter, 12 mm). The ampule was sealed at a length of 120 mm and heated to 450 °C (heating rate: 1 °C min-1). After about 5 h at this temperature, the ampule was slowly (2 °C min-1) cooled to room temperature. After the ampule was opened, the typical smell of ammonia was detected. At the top of the ampule, colorless crystals were found which were identified by X-ray powder diffractometry as sublimated melamine. At the bottom, a white-beige powder containing melem was isolated. Melem C6N7(NH2)3. yield 60%. Anal. calcd for melem: H, 2.75; C, 33.03; N, 64.22. Found: H, 2.98; C, 32.62; N, 62.04. |
| Spectrum Detail | Back Directory | [Spectrum Detail]
1,3,4,6,7,9,9b-heptaazaphenalene-2,5,8-triamine(1502-47-2)IR1
1,3,4,6,7,9,9b-heptaazaphenalene-2,5,8-triamine(1502-47-2)IR2
|
|
|