| Identification | Back Directory | [Name]
(S)-1-(5-fluoro-2-iodophenyl)ethan-1-ol | [CAS]
1454847-96-1 | [Synonyms]
EOS-60623
(S)-1-(2-Iodo-5-fluorophenyl)-ethanol
(S)-1-(5-fluoro-2-iodophenyl)ethan-1-ol
(1S)-1-(5-FLUORO-2-IODOPHENYL)ETHAN-1-OL
Benzenemethanol, 5-fluoro-2-iodo-α-methyl-, (αS)- | [Molecular Formula]
C8H8FIO | [MDL Number]
MFCD30803240 | [MOL File]
1454847-96-1.mol | [Molecular Weight]
266.051 |
| Hazard Information | Back Directory | [Uses]
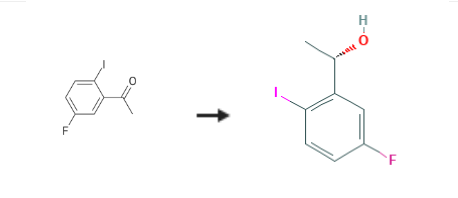
(S)-1-(5-fluoro-2-iodophenyl)ethan-1-ol is an intermediate of Lorlatinib, which is an orally administered inhibitor of ALK and ROS1, two enzymes that play a role in the development of cancer. | [Synthesis]
(S)-1-(5-fluoro-2-iodophenyl)ethan-1-ol is synthesised using 5'-Fluoro-2'-iodoacetophenone as a raw material by chemical reaction. The specific synthesis steps are as follows:
A solution of (-)-DIPCI (57.1 g, 178 mmol) in THF (100 ml) was cooled to -20 to -30 C. A solution of compound 1 (31 .3 g, 1 19 mmol) in THF (100 ml) was then added dropwise, via addition funnel (30 min addition). The reaction was left to warm up to RT. After 2 h, the reaction was cooled to -30 C and another portion of (-)-DIPCI (38.0 g, 1 19 mmol) was added. After 30 min, the reaction was allowed to warm to RT and after 1 h, the solvents were removed in vacuo and the residue re-dissolved in MTBE (200 ml). A solution of diethanolamine (31 g, 296 mmol) in ethanol/THF (15 ml/30 ml) was added via addition funnel, to the reaction mixture under an ice bath. The formation of a white precipitate was observed. The suspension was heated at reflux for 2 hours then cooled to room temperature, filtered and the mother liquids concentrated in vacuo. The residue was suspended in heptane/EtOAc (7:3, 200 ml) and again filtered. This procedure was repeated until no more solids could be observed after the liquids were concentrated. The final yellow oil was purified by column chromatography (eluent: cyclohexane/EtOAc- 99:1 to 96:4). The resulting colorless oil was further purified by recrystallisation from heptanes, to give alcohol compound 2 (25 g, 80% yield, 99% purity and 96% ee) as white crystals. 1H NMR (400 MHz, CDCI3) delta 7.73 (dd, 1 H), 7.32 (dd, 1 H), 6.74 (ddd, 1 H), 4.99 - 5.04 (m, 1 H), 2.01 (d, 1 H), 1.44 (d, 3 H). LCMS-ES: No ionization, Purity 99%. Chiral GC (column CP-Chirasil-DexnCB): 96% ee; Rt (minor) 17.7 minutes and Rt (major) 19.4 minutes.
 |
|
|