| Identification | Back Directory | [Name]
ETHYL 4-ACETYLBUTYRATE | [CAS]
13984-57-1 | [Synonyms]
Ethyl 5-oxohexanoate
ETHYL 4-ACETYLBUTYRATE
Ethyl 4-acetylbutanoate
Ethyl 4-acetylbutyrate 98%
5-oxo-hexanoicaciethylester
4-Acetylbutyricacidethylester
5-Oxohexanoic acid ethyl ester
5-Ketocaproic acid ethyl ester
Ethyl-4-acetylbutyrate, GC 98%
Hexanoic acid, 5-oxo-, ethyl ester | [EINECS(EC#)]
237-776-7 | [Molecular Formula]
C8H14O3 | [MDL Number]
MFCD00009213 | [MOL File]
13984-57-1.mol | [Molecular Weight]
158.19 |
| Chemical Properties | Back Directory | [Boiling point ]
221-222 °C(lit.)
| [density ]
0.989 g/mL at 25 °C(lit.)
| [refractive index ]
n20/D 1.427(lit.)
| [Fp ]
157 °F
| [storage temp. ]
Sealed in dry,Room Temperature | [solubility ]
Chloroform (Sparingly), Methanol (Slightly) | [form ]
Oil | [color ]
Colourless | [BRN ]
1762625 | [InChI]
InChI=1S/C8H14O3/c1-3-11-8(10)6-4-5-7(2)9/h3-6H2,1-2H3 | [InChIKey]
MGPSIDGTLFKDEY-UHFFFAOYSA-N | [SMILES]
C(OCC)(=O)CCCC(=O)C | [EPA Substance Registry System]
Ethyl 5-oxohexanoate (13984-57-1) |
| Hazard Information | Back Directory | [Uses]
5-Oxohexanoic Acid Ethyl Ester is a useful synthetic intermediate. It is used to prepare tyrosine-derived RGD peptidomimetics containing oligoethylene glycol (OEG) spacers. It is also used to synthesize constrained glycyl amides derived from RGD tripeptide as nonpeptide αvβ3 antagonists. | [Synthesis]
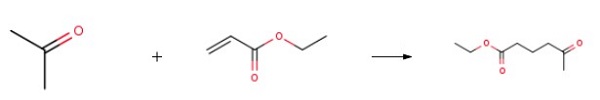
30ml of acetone and 11.5mmol of tetrahydropyrrole were added to a dry three-necked flask equipped with a thermometer and reflux condenser, and then slowly add 50mmol of ethyl acrylate dropwise at 60°C for 10h. After the reaction, the reaction solution is at 60°C Distill the remaining unreacted acetone and acidify with dilute hydrochloric acid to adjust the pH to 4. Add ethyl acetate for extraction, collect the organic layer, and perform preliminary vacuum distillation at 0.1MPa vacuum and 45°C to distill off the low-boiling solvent, followed by 3- Collect 70-80°C fractions under a vacuum of 7mmHg to prepare Ethyl 4-acetylbutyrate.
 |
|
|