| Identification | Back Directory | [Name]
(+)-1,2-BIS[(2S,5S)-2,5-DIMETHYLPHOSPHOLANO]BENZENE | [CAS]
136735-95-0 | [Synonyms]
(S,S)-ME-DUPHOS
(S,S)-METHYL-DUPHOS
(+)-1,2-BIS[(2S,5S)-2,5-DIMETHYLPHOSPHOLANO]BENZENE
1,2-Bis[(2S,5S)-2,5-diMethyl-1-phospholanyl]benzene, 97+%
(+)-1,2-Bis[(2S,5S)-2,5-diMethylphospholano]benzene kanata purity
(+)-1,2-Bis((2S,5S)-2,5-diMethylphospholano)benzene (S,S)-Me-DUPHOS
(2S,2'S,5S,5'S)-2,2',5,5'-TETRAMETHYL-1,1'-(O-PHENYLENE)DIPHOSPHOLANE
(+)-1,2-Bis((2S,5S)-2,5-dimethylphospholano)benzene,98+%(S,S)-Me-DUPHOS
(2S,2'S,5S,5'S)-2,2',5,5'-TETRAMETHYL-1,1'-(1,2-PHENYLENE)DIPHOSPHOLANE
(+)-1,2-Bis[(2S,5S)-2,5-dimethylphospholano]benzene,(2S,2′S,5S,5′S)-2,2′,5,5′-Tetramethyl-1,1′-(o-phenylene)diphospholane, (S,S)-Me-DUPHOS, (S,S)-Methyl-DUPHOS | [Molecular Formula]
C18H28P2 | [MDL Number]
MFCD00142322 | [MOL File]
136735-95-0.mol | [Molecular Weight]
306.36 |
| Chemical Properties | Back Directory | [Melting point ]
67-76 °C
| [alpha ]
D25 +476± 5° (c = 1 in hexane) | [Boiling point ]
415.0±15.0 °C(Predicted) | [storage temp. ]
Inert atmosphere,2-8°C | [form ]
crystal | [color ]
white | [Water Solubility ]
Insoluble in water. | [Sensitive ]
Air Sensitive | [BRN ]
4810602 |
| Questions And Answer | Back Directory | [Reaction]
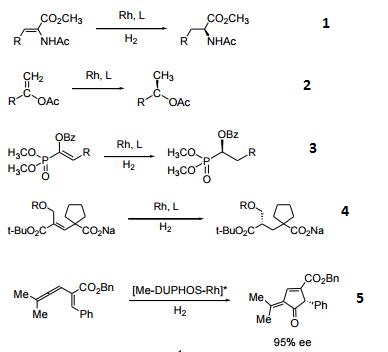
- The DUPHOS family of catalysts is highly efficient for the asymmetric hydrogenation of various substituted acetamidoacrylates and enol acetates yielding products of high enantiomeric excesses. Efficient ligand for the asymmetric hydrogenation of imines, enamines, and enamides.
- Asymmetric hydrogenation of vinyl alcohols.
- Catalyst used for the asymmetric hydrogenation of enol phosphonates.
- Asymmetric hydrogenation of allylic alcohols.
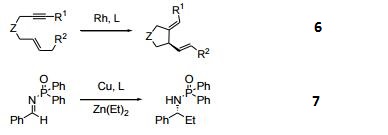
- Ligand for the catalytic asymmetric [4+1] cycloaddition of vinylallenes with CO.
- Ligand for the Rh-catalyzed asymmetric enyne cycloisomerization.
- Catalytic enantioselective addition of dialkylzinc to N-Diphenylphosphinoylimines.
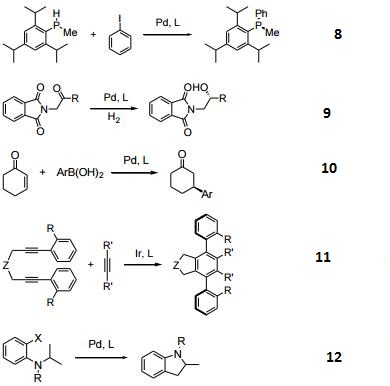
- Palladium-catalyzed asymmetric phosphination.
- Palladium-catalyzed asymmetric hydrogenation of carbonyls.
- Palladium-catalyzed 1,4 arylation of α, β-unsaturated ketones.
- Asymmetric, Ir-catalyzed, [2+2+2] cycloaddition.
- Asymmertric palladium-catalyzed synthesis of 2-methyl-indolines via C–H activation.
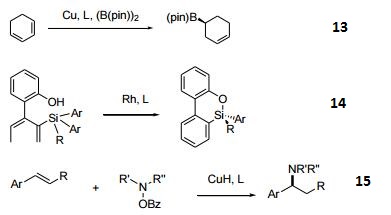
- Copper-catalyzed monoborylation of 1,3-Dienes.
- Rhodium-catalyzed enantioselective transmetalation.
- CuH-catalyzed hydroamination of styrenes.
|
|
| Company Name: |
ExSyn Corp
|
| Tel: |
+91-2240546474 +91-2240546474 |
| Website: |
www.exsyncorp.com |
| Company Name: |
Alfa Aesar
|
| Tel: |
400-6106006 |
| Website: |
http://chemicals.thermofisher.cn |
| Company Name: |
Energy Chemical
|
| Tel: |
021-021-58432009 400-005-6266 |
| Website: |
http://www.energy-chemical.com |
| Company Name: |
Sigma-Aldrich
|
| Tel: |
021-61415566 800-8193336 |
| Website: |
https://www.sigmaaldrich.cn |
|