| Identification | Back Directory | [Name]
Pyridine, 2-[(difluoroMethyl)sulfonyl]- | [CAS]
1219454-89-3 | [Synonyms]
2-[(DifL
Hu Reagent
2-PySO2CF2H
DifluoroMethyl 2-Pyridyl Sulfone
2-(Difluoromethanesulfonyl)pyridine
2-[(DifluoroMethyl)sulfonyl]pyridine
Pyridine, 2-[(difluoroMethyl)sulfonyl]-
Difluoromethyl 2-pyridyl sulfone 97% (HPLC) | [Molecular Formula]
C6H5F2NO2S | [MDL Number]
MFCD17010193 | [MOL File]
1219454-89-3.mol | [Molecular Weight]
193.171 |
| Chemical Properties | Back Directory | [Melting point ]
47.0 to 51.0 °C | [Boiling point ]
324.4±42.0 °C(Predicted) | [density ]
1.423±0.06 g/cm3(Predicted) | [storage temp. ]
-20°C Freezer, Under inert atmosphere | [solubility ]
Chloroform (Slightly), Methanol (Slightly) | [form ]
Solid | [pka]
-2.60±0.19(Predicted) | [color ]
Off-White | [BRN ]
20317604 |
| Hazard Information | Back Directory | [Description]
Difluoromethyl 2-pyridyl sulfone, also known as Hu reagent, is a novel and efficient gem-difluoroolefination reagent for preparing gem-difluoroalkenes from both aldehydes and ketones. The fluorinated sulfinate intermediates during the gem-difluoroolefination is relatively stable, and can be halogenated in situ to afford bromo- and iododifluoromethyl compounds. It can also act as nucleophilic difluoro(sulfonato)methylation reagent for the synthesis of α,α-difluorosulfonates from aldehydes, and alkyl halides and triflates. | [Uses]
2-[(Difluoromethyl)sulfonyl]pyridine is a new novel gem-difluoroolefination reagent for both aldehydes and ketones. | [Reactions]
(1) gem-Difluoroolefination of aldehydes and ketones.
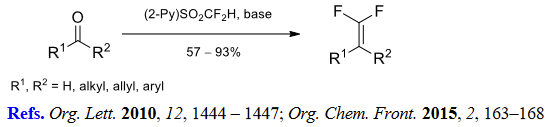
(2) Halodifluoromethylation of aldehydes and ketones.
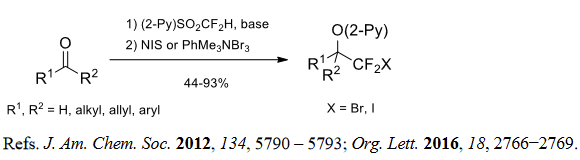
(3) (Fluorosulfonyl)difluoromethylation of aldehydes and ketones.
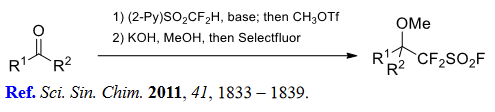
(4) Difluoro(sulfonato)methylation of alkyl halides and triflates.
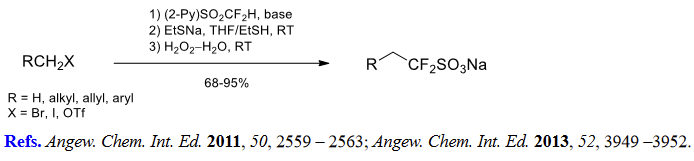
(5) Aromatic difluoromethylation
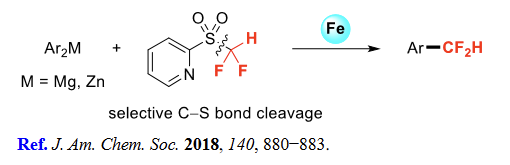 | [General Description]
Difluoromethyl 2-pyridyl sulfone (2-PySO2CF2H) is a reagent used in the gem-difluoroolefination of aldehydes and ketones. It is also used as a reagent in the nucleophilic difluoro(sulfonato)methylation of alcohols, N-sulfinyl imines, and halides. | [References]
[1] WENJUN MIAO. Nickel-Catalyzed Reductive 2-Pyridination of Aryl Iodides with Difluoromethyl 2-Pyridyl Sulfone[J]. Organic Letters, 2021, 23 3: 711-715. DOI:10.1021/acs.orglett.0c03939.
[2] WENJUN MIAO. Nucleophilic Iododifluoromethylation of Carbonyl Compounds Using Difluoromethyl 2-Pyridyl Sulfone[J]. Organic Letters, 2016, 18 11: 2766-2769. DOI:10.1021/acs.orglett.6b01258.
[3] YANCHUAN ZHAO. Difluoromethyl 2-Pyridyl Sulfone: A New gem-Difluoroolefination Reagent for Aldehydes and Ketones[J]. Organic Letters, 2010, 12 7: 1444-1447. DOI:10.1021/ol100090r. |
|
|