| Identification | Back Directory | [Name]
BrettPhosPalladacycle | [CAS]
1148148-01-9 | [Synonyms]
BrettPhos precatalyst
BrettPhos Palladacycle
BrettPhos Pd G1 Methyl-t-Butyl Ether Adduct
(BrettPhos) palladium(II) phenethylamine chloride
Chloro[2-(dicyclohexylphosphino)-3,6-dimethoxy-2',4',6'-triisopropyl-1,1'-biphenyl][2-(2-amino
Chloro[2-(dicyclohexylphosphino)-3,6-dimethoxy-2',4',6'-triisopropyl-1,1'-biphenyl][2-(2-aminoeth
Chloro[2-(dicyclohexylphosphino)-3,6-dimethoxy-2'-4'-6'-triisopropyl-1,1'-biphenyl][2-(2-aminoethyl)phenyl]palladium(II)
Chloro[2-(dicyclohexylphosphino)-3,6-dimethoxy-2'-4'-6'-tri-i-propyl-1,1'-biphenyl][2-(2-aminoethyl)phenyl]palladium(ii)
Chloro[2-(dicyclohexylphosphino)-3,6-diMethoxy-2'-4'-6'-tri-i-propyl-1,1'-biphenyl][2-(2-aMinoethyl)phenyl]palladiuM(II),98%
Dicyclohexyl(2',4',6'-triisopropyl-3,6-dimethoxy-[1,1'-biphenyl]-2-yl)phosphine-(2-(2-aminoethyl)phenyl)palladium(II) chloride
Chloro[2-(dicyclohexylphosphino)-3,6-diMethoxy-2'-4'-6'-tri-i-propyl-1,1'-biphenyl][2-(2-Minoethyl)phenyl]palladiuM(II),Min.98%[BrettPhosPalladacycle]
Chloro[2-(dicyclohexylphosphino)-3,6-diMethoxy-2',4',6'-tri-i-propyl-1,1'-biphenyl][2-(2-aMinoethyl)phenyl]palladiuM(II), Min. 98% [BrettPhos Palladacycle] | [Molecular Formula]
C43H63ClNO2PPd | [MDL Number]
MFCD23843793 | [MOL File]
1148148-01-9.mol | [Molecular Weight]
797.805 |
| Questions And Answer | Back Directory | [Reaction]
- Catalyst for cross-coupling reactions using aryl mesylates with electron-deficient anilines.
- Catalyst for rapid C-N bond-forming processes at low catalyst loading.
- Cross-coupling of 3-Bromo-2-aminopyridine with primary amines.
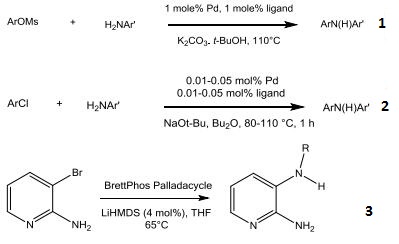
|
| Hazard Information | Back Directory | [Uses]
| [General Description]
BrettPhos Pd G1 is a Buchwald first generation (G1) palladacycle precatalyst that can be used in cross-coupling reactions for the formation of C-C, C–N, C–O, C–F, C–CF3, and C–S bonds. The formation of the active Pd(0) species in the G1 precatalyst is easy to generate, requiring merely a deprotonation with a base. The resulting catalyst is very active, even at temperatures down to ?40 °C, and can be used in a variety of cross-coupling protocols. |
|
|