| Identification | Back Directory | [Name]
CAL-101 | [CAS]
1146702-54-6 | [Synonyms]
Idelalisib
GS-1101, Idelalisib
CAL-101 (GS-1101, Idelalisib)
5-fluoro-3-phenyl-2-((1s)-1-(1h-purin-6-ylamino)ethyl)-4(3h)... | [Molecular Formula]
C22H18FN7O | [MOL File]
1146702-54-6.mol | [Molecular Weight]
415.423 |
| Hazard Information | Back Directory | [Description]
Idelalisib is an orally available selective and potent phosphatidylinositol
3-kinase δ (PI3 Kδ) inhibitor originally developed
by Calistoga Pharmaceuticals, which was acquired by Gilead in
April 2014. In July 2014, the drug was approved in the USA for
the treatment of relapsed chronic lymphocytic leukemia as well
as several oncology orphan drug designations. Since idelalisib
specifically inhibits PI3Kd, which is expressed primarily in bloodcell
lineages, the therapeutic effect is localized, limiting interference
with PI3K isoform signaling that is critical to normal function
of healthy cells. | [Definition]
ChEBI: Idelalisib is a member of the class of quinazolines that is 5-fluoro-3-phenylquinazolin-4-one in which the hydrogen at position 2 is replaced by a (1S)-1-(3H-purin-6-ylamino)propyl group. used for for the treatment of refractory indolent non-Hodgkin's lymphoma and relapsed chronic lymphocytic leukemia. It has a role as an antineoplastic agent, an apoptosis inducer and an EC 2.7.1.137 (phosphatidylinositol 3-kinase) inhibitor. It is a member of purines, an organofluorine compound, a member of quinazolines, an aromatic amine and a secondary amino compound. | [Indications]
Among the large groups of structural diverse lipid kinase inhibitors, especially against PI3Ks, idelalisib (Zydelig(R), Gilead Sciences) is the only inhibitor approved by FDA for the treatment of patients with relapsed chronic lymphocytic leukemia in combination with rituximab and patients with relapsed follicular B-cell non-Hodgkin lymphoma or small lymphocytic lymphoma. | [Brand name]
Zydelig | [General Description]
Class: lipid kinase;
Treatment: CLL, SLL, FL;
Other name: CAL-101, GS-1101;
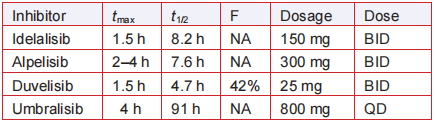
Elimination half-life = 8.2 h;
Protein binding > 84% | [Pharmacokinetics]
The recommended dose of idelalisib is 150 mg
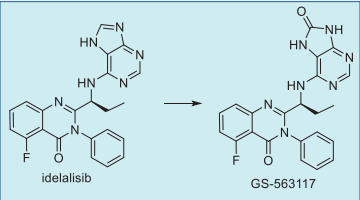
orally twice a day, consistent with its elimination halflife is 8.2 h (Table 2). It is absorbed rapidly with a tmax of 1.5 h. Idelalisib is metabolized by aldehyde
oxidase and CYP3A to give a major metabolite GS-
563117 (Fig. 7), which is inactive against P110δ and
other isoforms.
  | [Clinical Use]
Phosphatidylinositol 3-kinase p110δ (PI3Kδ) inhibitor:
Treatment of chronic lymphocytic leukaemia (CLL)
and follicular lymphoma (FL) | [Synthesis]
Commercial 2-fluoro-6-nitrobenzoic acid (117) was treated
with oxalyl chloride in the presence of catalytic amount of N,Ndimethylformamide
(DMF) in DCM to give the corresponding 2-
fluoro-6-nitrobenzoyl chloride as a brown syrup, which was subsequently
coupled with aniline under Schotten-Baumann conditions
to yield 2-fluoro-6-nitro-N-phenylbenzamide 118 in 99% yield.
Coupling of 118 with commercial N-Boc-2(S)-aminobutyric acid
in the presence of Et3N in DCM generated imide 119 in 66% yield.
Reductive cyclization of nitro imide 119 by means of zinc dust in
acetic acid gave the cyclized quinazolinone 120 in 69% yield, which
underwent immediate N-deprotection with TFA in DCM to furnish
the corresponding free amine 121. Finally, a substitution reaction
involving amine 121 and 6-bromopurine (122) in the presence of
DIPEA in t-BuOH gave idelalisib (XV) as a solid in 50% yield.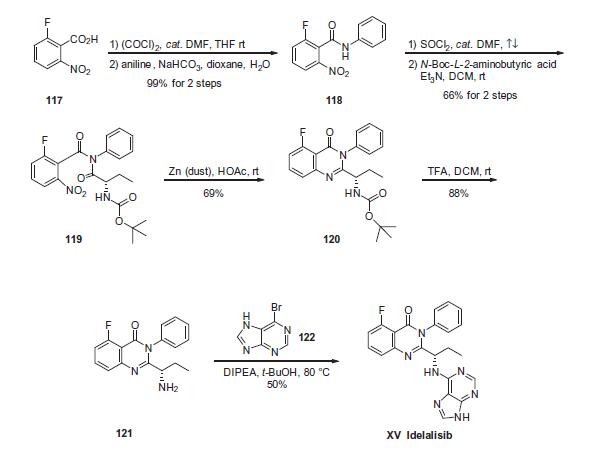
| [target]
PI3Kδ | [Drug interactions]
Potentially hazardous interactions with other drugs
Antibacterials: concentration reduced by rifampicin
- avoid.
Antidepressants: concentration possibly reduced by
St John’s wort - avoid.
Antiepileptics: concentration possibly reduced by
carbamazepine, fosphenytoin and phenytoin - avoid.
Antipsychotics: avoid with clozapine, increased
risk of agranulocytosis; avoid with pimozide and
quetiapine. | [IC 50]
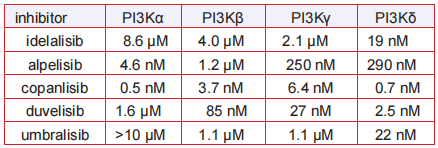 | [Metabolism]
Idelalisib is metabolised mainly via aldehyde oxidase, and
to a lesser extent via CYP3A and UGT1A4. The primary
and only circulating metabolite, GS-563117, is inactive
against PI3Kδ.
Following a single 150 mg oral dose of [14C]-labelled
idelalisib, approximately 78% and 15% was excreted
in faeces and urine, respectively. Unchanged idelalisib
accounted for 23% of total radioactivity recovered in urine
over 48 hours and 12% of total radioactivity recovered in
faeces over 144 hours. |
|
| Company Name: |
NCE Biomedical Co.,Ltd.
|
| Tel: |
4000-027-021 |24 +86-13986109188 | +86-15623472865 | +81-08033611988 |
| Website: |
www.approvedhomemanagement.com/ShowSupplierProductsList15748/0.htm |
| Company Name: |
SPIRO PHARMA
|
| Tel: |
|
| Website: |
www.spiropharma.com.cn |
|