| Identification | Back Directory | [Name]
(S)-2-HYDROXYMETHYL-PIPERAZINE-1-CARBOXYLIC ACID TERT-BUTYL ESTER | [CAS]
1030377-21-9 | [Synonyms]
(S)-1-Boc-2-(hyroxyMethyl)piperazine
(S)-N1-BOC-2-Hydroxymethyl-piperazine
(S)-1-N-BOC-2-HYDROXYMETHYLPIPERAZINE
(2S)-2-(Hydroxymethyl)piperazine, N1-BOC protected
(S)-tert-butyl 2-(hydroxyMethyl)piperazin-1-carboxylate
(S)-tert-butyl 2-(hydroxyMethyl)piperazine-1-carboxylate
tert-butyl (S)-2-(hydroxymethyl)piperazine-1-carboxylate
tert-butyl (2S)-2-(hydroxymethyl)piperazine-1-carboxylate
1,1-Dimethylethyl (2S)-2-(hydroxymethyl)-1-piperazinecarboxylate
(S)-2-HYDROXYMETHYL-PIPERAZINE-1-CARBOXYLIC ACID TERT-BUTYL ESTER
(2S)-2-(hydroxymethyl)-1-Piperazinecarboxylic acid tert-butyl ester
1-PIPERAZINECARBOXYLIC ACID, 2-(HYDROXYMETHYL)-, 1,1-DIMETHYLETHYL ESTER, (2S)-
tert-Butyl (2S)-2-(hydroxymethyl)piperazine-1-carboxylate, (2S)-1-(tert-Butoxycarbonyl)-2-(hydroxymethyl)piperazine | [Molecular Formula]
C10H20N2O3 | [MDL Number]
MFCD07772093 | [MOL File]
1030377-21-9.mol | [Molecular Weight]
216.28 |
| Chemical Properties | Back Directory | [Boiling point ]
324℃ | [density ]
1.085 | [Fp ]
150℃ | [storage temp. ]
Keep in dark place,Inert atmosphere,2-8°C | [pka]
14.97±0.10(Predicted) | [Appearance]
White to off-white Solid | [InChI]
InChI=1S/C10H20N2O3/c1-10(2,3)15-9(14)12-5-4-11-6-8(12)7-13/h8,11,13H,4-7H2,1-3H3/t8-/m0/s1 | [InChIKey]
BCPPNDHZUPIXJM-QMMMGPOBSA-N | [SMILES]
N1(C(OC(C)(C)C)=O)CCNC[C@H]1CO |
| Hazard Information | Back Directory | [Reactions]
(S)-1-Boc-2-(Hydroxymethyl)piperazine reacts with 1,2-difluoro-4-nitrobenzene to synthesize tert-butyl (S)-4-(2-fluoro-4-nitrophenyl)-2-(hydroxymethyl)piperazine-1-carboxylate. This compound serves as a crucial intermediate in the preparation of the Wee-1 kinase activity inhibitor.
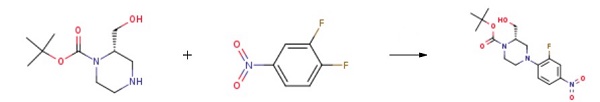 | [Synthesis]
To a solution of (S)-4-benzyl 1-tert-butyl 2-(hydroxymethyl)piperazine-1,4-dicarboxylate (1.5 g, 4.28 mmol) in MeOH (20 mL), 10 % Pd/C (150 mg) was added. The mixture was stirred at room temperature for 3 hours under H2. Afterwards, the solid was removed by filtration and the filtrate was concentrated to yield (S)-1-Boc-2-(Hydroxymethyl)piperazine (850 mg, 3.93 mmol, 91.82 percent) as a white solid.
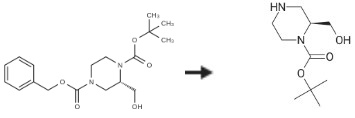
| [References]
[1] Patent: US2018/127370, 2018, A1. Location in patent: Paragraph 1006; 1004 |
|
|