| Identification | Back Directory | [Name]
1H-PYRROLO[2,3-B]PYRIDIN-5-YLAMINE | [CAS]
100960-07-4 | [Synonyms]
5-AMINO-7-AZAINDOLE
3-B]PYRIDIN-5-YLAMINE
5-AMino-7-aza-1H-indole
5-Amino-7-azaindole,97%
1H-PYRROLO[2,3-B]PYRIDIN-5-AMINE
1H-PYRROLO[2,3-B]PYRIDINE-5-AMINE
5-Amino-1H-pyrrolo[2,3-b]pyridine
1H-PYRROLO[2,3-B]PYRIDIN-5-YLAMINE
1H-Pyrrolo[2,3-b]pyridine,5-amino-(6CI)
1H-PYRROLO[2,3-B]PYRIDIN-5-YLAMINE ISO 9001:2015 REACH
1H-Pyrrolo[2,3-b]pyridin-5-ylaMine | [Molecular Formula]
C7H7N3 | [MDL Number]
MFCD06659683 | [MOL File]
100960-07-4.mol | [Molecular Weight]
133.15 |
| Chemical Properties | Back Directory | [Melting point ]
128-129°C | [Boiling point ]
361.7±35.0 °C(Predicted) | [density ]
1.41±0.1 g/cm3(Predicted) | [storage temp. ]
Keep in dark place,Sealed in dry,Room Temperature | [form ]
Solid | [pka]
8.28±0.20(Predicted) | [color ]
Yellow to yellow/brown | [Sensitive ]
Light Sensitive | [InChIKey]
PLWBENCHEUFMTN-UHFFFAOYSA-N | [CAS DataBase Reference]
100960-07-4 |
| Hazard Information | Back Directory | [Chemical Properties]
Brown crystalline powder | [Synthesis]
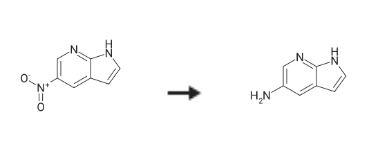
5-Amino-7-azaindole is synthesised using 5-Nitro-7-azaindole as a raw material by chemical reaction. The specific synthesis steps are as follows:
To a stirred solution of 5nitro7azaindoie (500 rng, 3.07 rnmol) in methanol (125 rnL) under nitrogen atmosphere was added 10percent Pd/C (326 mg, 0.306 rnmol). The reaction was placed under an atmosphere of hydrogen gas and stirred at 20 °C overnight. The suspension was filtered through a pad of Celite? 545, the resulting filter cake was washed with MeOH, and the combined organicphases concentrated in vaeuo to afford 5-Amino-7-azaindole compound (408 mg, 100percent). MS (ES1): mass calcd. for C7HN3 133.1, rn/z found 133.9 [M+H]t. ‘H NMR (300 MHz, DMSO-d6) ? 11.04 (s, 1H), 7.70 (d, J= 2.4 Hz, iH, 7.27 —7.18 (m, 1ff), 7.08 (d, J= 2.3 Hz, IH), 6.22 —6.06 (m, 1H), 4.62 (s, 2H).
 |
|
|