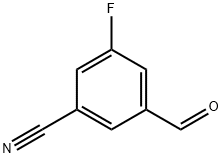
| Identification | Back Directory | [Name]
3-FLUORO-5-FORMYLBENZONITRILE | [CAS]
1003708-42-6 | [Synonyms]
3-FLUORO-5-FORMYLBENZONITRILE
Benzonitrile, 3-fluoro-5-formyl- | [Molecular Formula]
C8H4FNO | [MDL Number]
MFCD04974123 | [MOL File]
1003708-42-6.mol | [Molecular Weight]
149.12 |
| Hazard Information | Back Directory | [Uses]
3-Fluoro-5-formylbenzonitrile is benzaldehyde based building block used as a reactant in catalyst-free synthesis of N-β-hydroxyethyl pyrroles and indoles via a domino [3+2] cycloaddition and ring-opening aromatization process. | [Application]
3-FLUORO-5-FORMYLBENZONITRILE can be used as organic synthesis intermediate and pharmaceutical intermediate, mainly used in laboratory research and development process and chemical production process. | [Synthesis]
3 -fluoro-5 -formylbenzonitrile : A solution of 3 -bromo-5 -fluorobenzonitrile(5.00 g, 25.0 mmol) in dry THF (25 mL) was cooled to 0 °C and 2M iPrMgCl (15.0 mL, 30.0 mmol) in THF was added dropwise over 5 minutes. The mixture was stirred at 0 °C for 15 minutes then at ambient temperature for 1 hour. The mixture was cooled to 0 °C and dry DMF (5.81 mL, 75.0 mmol) was added. The mixture was stirred for 17 hours during which time the temperature reached ambient temperature after 2 hours. The mixture was added to ice water (150 mL) and Et20 (100 mL). The biphasic mixture was stirred and treated with 6M HC1 to aqueous pH=3. The organic layer was removed and the aqueous layer extracted with Et20 (2X). The combined Et20 fractions were washed with saturated NaCl and dried over MgS04/activated carbon. The dried solution was filtered through a S1O2 plug eluting with Et20. The filtrate was concentrated to give the title compound as a yellow solid that was dried in vacuum (3.68 g, 99percent). 1H NMR (CDC13) δ 10.0 (s, 1H), 8.00 (s, 1H), 7.81-7.86 (m, 1H), 7.62-7.67 (m, 1H). |
|
|